There is a lot of reports on technologies that have already entered the mainstream and will continue to grow in 2018 such as T-cell receptor targeted cancer, autoimmune and gene therapies. European Biotechnology Magazine takes a look on what really is new and has the potential to change current medical paradigms.
ADVERTISEMENT
It’s still under the radar of analysts. However, the vasoactive peptide adrenomedullin could become the Next Big Thing in therapy monitoring and the treatment of disorders caused by endothelial dysfunction such as acute heart failure, or septic shock. During a scientific symposium Endothelial Dysfunction – Adrenomedullin as a diagnostic and therapeutic target (19. December 2017, Berlin), 15 international medical key opinion leaders from ICU/ED’s for the first time compiled clinical data from more than 10,000 patients that underscore that adrenomedullin could lead to a paradigm shift in the diagnosis, monitoring and treatment of disorders linked to endothelial dysfunction.
Therapy-induced senescence (TIS), a lasting chemotherapy-evoked proliferative arrest of tumor cells, has been thought to be irreversible. As it turns out now, it makes tumour cells, which survive, more aggressive and worsens prognosis.
Scientists have generated sheets of human eye cells arranged on a biological scaffold, which they used to successfully treat vision disorders in rats.
The European Commission has closed its very first infringement procedure under the Penalty Regulation. During an inspection at Roche’s UK headquarters in 2012 the British MHRA found data on 80,000 not properly reported adverse events of approved Roche meds, 15,161 of which included deaths.
Hadean Ventures announced the launch of Hadean Capital I, a fund that will invest up to € 100 m in life science companies, with a particular focus on the Nordic region.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) recommended seven medicines for approval at its December 2017 meeting, including two orphan medicines, and one biosimilar.
Stevenage Bioscience Catalyst (SBC) has announced that Dr. Sally Ann Forsyth is set to join the company as CEO in March 2018.
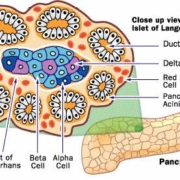
Belgian reMYND N.V. has out licenced worldwide commercialisation rights for its small molecule programme ReS39 in diabetes to Novo Nordisk A/S. The drug impedes death of insulin-producing beta cells by preventing the formation of cytotoxic islet amyloid polypeptide (IAPP) aggregates.
A new, non-invasive tuberculosis urine test prevents common disadvantages of the standard tuberculin skin test: Measuring lipoarabinomannan glycan from M. tuberculosis, it detects the active disease in early stage and does not interfere with co-infections or weakened immune system.