Novel COVID antibody enters clinical testing
German CORAT Therapeutics GmbH got the greenlight for Phase Ib/II testing of COR-101, an antibody that reduced virus load in the lung by more than 99 % within three days.
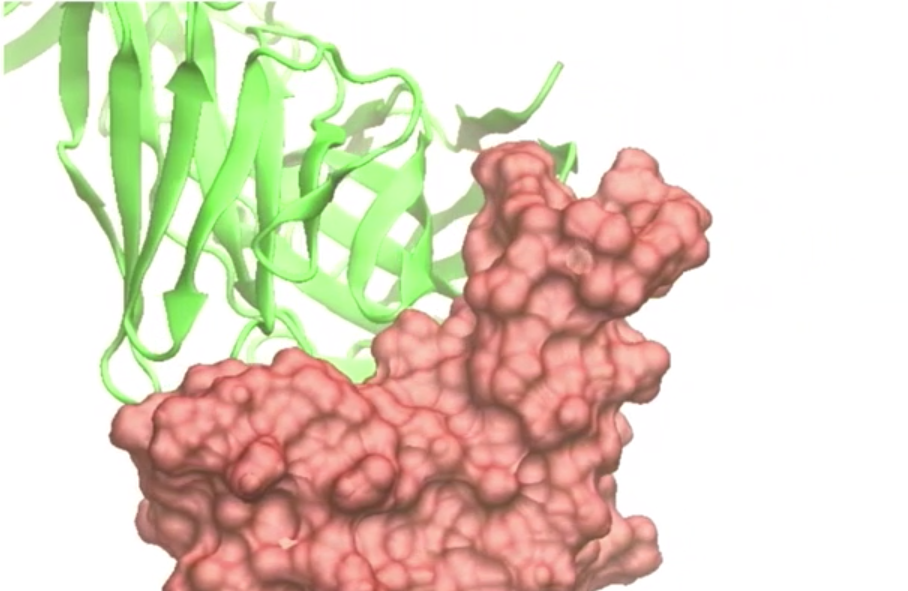
In contrast to existing COVID-19 treatments, the safety design of COR-101 is optimised for the treatment of hospitalized COVID-19 patients with moderate to severe disease. According to CORAT Therapeutics, COR-101 binds to the receptor binding domain (RBD) of the spike protein of SARS-CoV-2 and is endowed with a unique structural design. It is designed to improve the treatment situation of hospitalized COVID-19 patients with moderate to severe disease, a patient group which currently cannot be treated appropriately due to the lack of effective medication.
The design of COR-101 prevents any Fc-gamma receptor binding and thus avoids the risk of antibody dependent enhancement (ADE) of viral proliferation. According to the company, the antibody binds to many mutated variants of SARS-CoV-2, such as the British variant, B.1.1.7., but also to the new and rapidly spreading "Czech (N439K) or "New York and "Nigeria" variants (E484K).
"We feel confident that COR-101 can fill the gap in the medical need for the treatment of hospitalized COVID-19 patients that have moderate and severe symptoms, where no other specific treatment is available at this time worldwide," said Dr. Andreas Herrmann, CEO of CORAT Therapeutics. "The development of specific therapeutics to treat COVID-19 diseases is next to vaccination and testing an important pillar to effectively fight the pandemic."
The study (ClinicalTrials.gov ID: NCT04674566) is a randomized, double-blind, placebo-controlled, parallelgroup, first-in-human, phase Ib/II study to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, immunogenicity, and efficacy of COR-101 in hospitalized patients with moderate to severe COVID-19. The study will start in six study centers in Germany and expanded up to 15 study centers in Europe to give fast access to the patients. The overall medical responsibility for the study lies with Prof. Dr. Helmut Salih at the University Hospital in Tuebingen.


 Unsplash+
Unsplash+