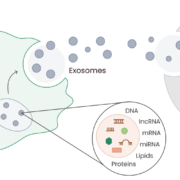
Exosomes are extracellular vesicles ranging from 30 to 150 nm in diameter that are released from various types of cells, including tumor cells. They can induce apoptosis, modulate the immune system, and function as biomarkers for diagnosis. In addition, as an important component of cell-to-cell communication, exosomes can regulate the tumor microenvironment and are involved in the development, progression, and metastasis processes of numerous cancers1,2.
ADVERTISEMENT
Tag Archive for: sino biological
Sino Biological, a biotechnology company listed on the Shenzhen stock exchange subsidiary ChiNext (SZSE: 301047), which provides biological research reagents and related technical contract research services, is pleased to announce the formal signing of a lease with and initiation of construction on its new Center for Bioprocessing (C4B) at its facility in Houston, Texas USA.
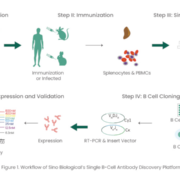
Single B cell screening is a powerful and efficient strategy for generating antigen-specific monoclonal antibodies (mAbs).
Since the approval of Orthoclone OKT3 in 1986, more than 100 monoclonal antibodies (mAbs) have been approved by the Food and Drug Administration (FDA) to treat a variety of diseases ranging from autoimmune disorders, infectious diseases, and cancer [1-2]. In the context of the current COVID-19 pandemic, it´s crucial that these biologics are developed rapidly and efficiently. Among various antibody discovery approaches, including hybridoma technology, single B cell screening is a powerful and efficient strategy for generating antigen-specific mAbs based on the direct amplification of the VH and VL regions encoding genes from single B cells [3-4]. Notably, single B cell screening has various advantages that include maintaining the naïve VH/VL pairing, requiring relatively few cells, and the ability to discover antibodies against challenging targets.
Nanobodies are emerging as important tools for tumor diagnosis and treatment due to their small size, simple humanization, low immunogenicity, superior affinity and stability, high penetration, and adequate levels of solubility, and are expected to revolutionize the antibody-based drug therapy. In 2018, the world´s first nanobody was approved for marketing in the European Union to treat adult patients with acquired thrombotic thrombocytopenic purpura. At present, more than 20 nanobodies are being tested in clinical research worldwide, mostly in clinical phase I or II.
An anti-idiotypic antibody (anti-ID Ab) can recognise and bind to the idiotypic center of another antibody. Anti-idiotype antibodies are widely used in therapeutic drug development, such as immunogenicity assessments, pharmacokinetic/pharmacodynamics (PK/PD) studies, and vaccine development.
Sino Biological, a biotechnology company listed on the Shenzhen stock exchange subsidiary ChiNext (SZSE: 301047), which provides biological research reagents and related technical is pleased to announce a CRO services partnership with Ainnocence, Inc. of San Jose, California.
Sino Biological specializes in recombinant production of antibodies and proteins. The company routinely conducts high-throughput projects of up to 1,000 per batch. Sino Biological’s manufacturing facility can also handle large-scale production at gram level.
Sino Biological, Inc., a biotechnology company listed on ChiNext (SZSE: 301047), provides biological research reagents and related technical contract research services, is pleased to announce its physical geographic expansion, adding a new location in Houston, Texas.
Sino Biological, Inc. has recently deposited reagents for the Omicron variant, including recombinant proteins to Spike and Nucleocapsid, and antibody products to a central repository for reagents to support infectious disease research. With the addition of the Omicron products to previously deposited array of SARS-CoV-2 reagents, researchers can readily get access to them by registering with BEI Resources.