Paris-based Klineo SAS has raised €2M to speed up access to clinical trials for oncology patients using an AI platform.
`
ADVERTISEMENT
Tag Archive for: Clinical trials
With a new law, the German government wants to increase the number of clinical trials conducted in Germany and make the country number one in Europe again.
Born with the aim of harmonising the application, evaluation, and supervision process of clinical trials conducted in EU countries, there is much room for improvement for the Clinical Trial Information System CTIS, particularly with regard to SMEs and translational researchers.
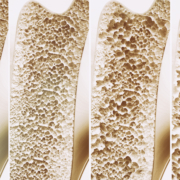
AVT03, a biosimilar candidate for osteoporosis treatment, showed promise in treating bone illnesses, according to results from a pharmacokinetic study by Alvotech SA.
Clinical trials have to be coordinated as a safe process where monitoring, analytics and handling of patient samples is streamlined – even cross oceans. German MLM Medical Labs is quickly establishing itself as an emerging central and specialty lab in the U.S.
Depending on who you talk to, artificial intelligence (AI) is either a saviour or an enslaver. The concerns range from loss of jobs through to robots running society. However, in the life sciences, the use of AI and machine learning (ML) have created some excitement over their potential applications.
A new platform is set to facilitate the exchange of information on the optimisation of the electronic reporting system CTIS.