Single-use bioreactor technology offers a set of benefits compared to common glass or stainless-steel vessels, including reduced downtime between runs, minimal cleaning effort, or decreased cross-contamination risk. In that regard, BioBLU® Single-Use Bioreactors combine the benefits of single-use bioreactor technology with the reliable performance of conventional glass or stainless-steel bioreactors. Furthermore, using them together with a powerful bioprocess controller like the BioFlo® 320 enables precise cell culture parameter control and seamless process transition to up to 40 L of working volume.
ADVERTISEMENT
Tag Archive for: biomanufacturing
Danish industrial biotechnology startup Cellugy raises €4,9m with the ambition to replace petrochemicals in personal care products.
Danish CDMO 21st.BIO A/S has unveiled a new pilot plant facility to accelerate scaling of biotech processes ranging from food and beverages, agriculture, biomaterials, and biopharma.
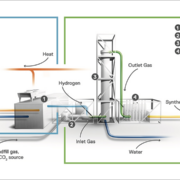
Archaeal Power-to-biomethan producer Electrochaea GmbH has sold a BioCat bioreactor to the Danish CCU specialist Again A/S.
Finnish Nordic Bioproducts Group has opened a production facility to produce advanced cellulosic materials wit the start-up’s AaltoCell technology
Professionalism and quality are always at the forefront along the whole value chain: from gene to product. To pro-actively meet the evolving demand of the market to produce new assets Richter-Helm significantly increased its manufacturing capacities for biopharmaceutical products at its production site in Bovenau, Germany.
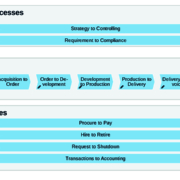
The biotech and pharma branch is growing steadily. Many companies have full order books for the next few years and expect growth. This is the basis for new investments in assets and infrastructure. These growing structures often expose vulnerabilities and challenges for the business. Therefore, companies need to increase the stability and efficiency of their processes.
Within the competitive CDMO market, Richter-Helm has developed into a highly valued partner when it comes to the manufacturing of biopharmaceuticals. Richter-Helm is well recognised by pharmaceutical companies, including Big Pharma, as a professional partner offering flexible CDMO solutions from gene to product, all from one source, including consultancy services. This makes Richter-Helm one of the key players in CDMO business.
The UK is making €15m investment in its biopharmaceutical manufacturing industry via its national innovation agency Innovate UK. It is part of the country’s larger push to support the domestic Life Sciences.