ADC Applications Beyond Oncology
Antibody-Drug Conjugates (ADCs) have been transformative in oncology, offering targeted cancer treatments that enhance efficacy while sparing healthy cells. However, the potential of ADCs extends well beyond cancer care. From autoimmune diseases to metabolic disorders, advancements in ADC technology are paving the way for breakthroughs across a range of therapeutic areas. This article explores how ADCs are redefining treatment paradigms and improving patient outcomes in diverse fields of medicine.
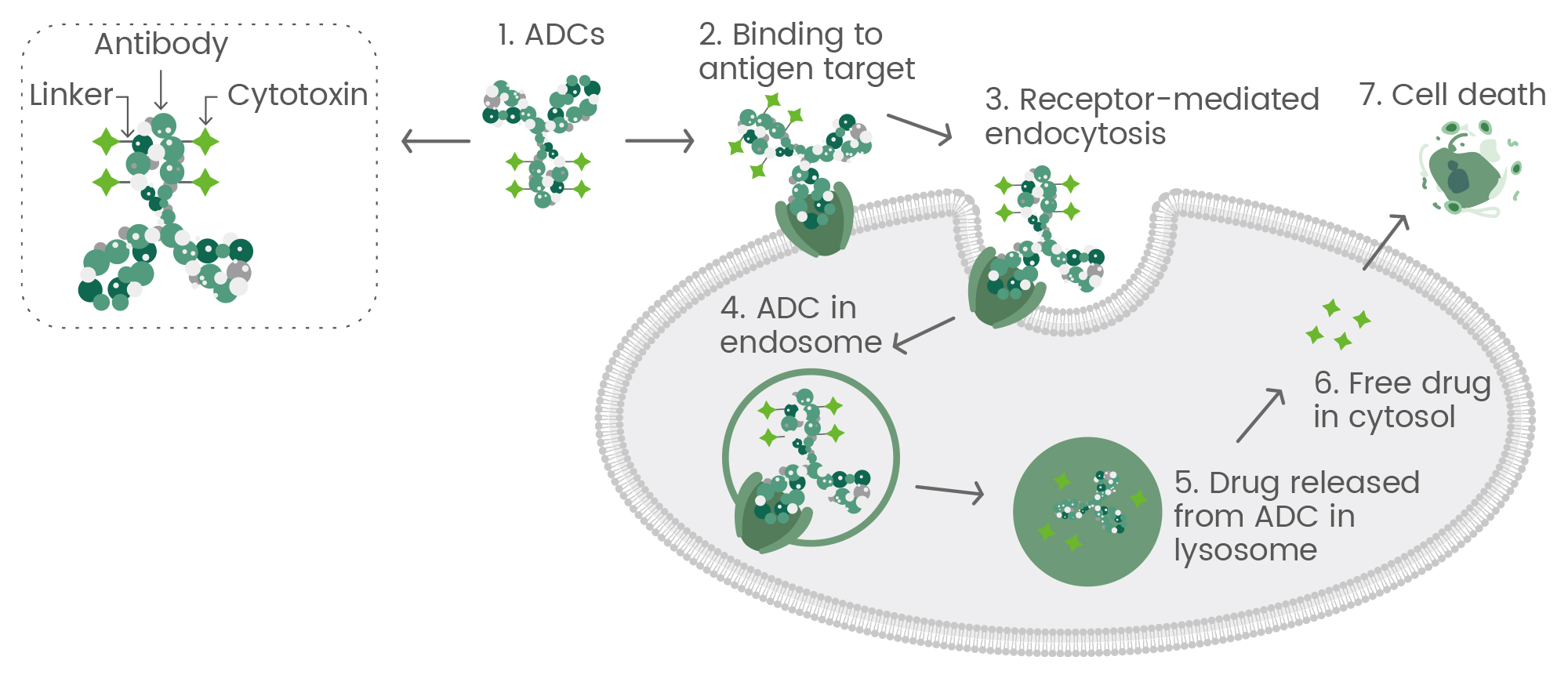
ADCs are a groundbreaking class of therapeutics that combine monoclonal antibodies with cytotoxic drugs, connected through chemical linkers. The monoclonal antibody specifically binds to antigens on diseased cells, allowing the drug to be delivered precisely where it is needed. Once internalized by the target cell, the linker is cleaved, releasing the cytotoxic drug to exert its therapeutic effect1,3. Originally developed for cancer, ADCs have demonstrated a unique ability to target diseases at a molecular level, offering precision and reduced toxicity compared to traditional therapies2.
With advancements in payload engineering, linker technology, and antigen discovery, ADCs are now inspiring innovation across therapeutic areas that include autoimmune diseases, inflammatory conditions, infectious diseases, neurological disorders, and metabolic diseases2–4.
Figure 1. The general mechanism of action for antibody-drug conjugates (ADCs).
Autoimmune Diseases: Precision in Immune Modulation
Autoimmune diseases have emerged as a significant focus for non-cancer ADC development. Conditions such as rheumatoid arthritis, lupus, psoriasis, ulcerative colitis, and scleroderma are often driven by dysregulated immune responses that attack the body’s own tissues. Traditional immunosuppressive therapies, while effective, come with significant side effects due to their lack of specificity.
In contrast, ADCs provide a highly targeted strategy for autoimmune diseases such as rheumatoid arthritis (RA). For example, AbbVie’s ABBV-3373 combines adalimumab (an anti-TNF monoclonal antibody) with a glucocorticoid receptor modulator (GRM). This ADC delivers the GRM payload specifically to immune cells expressing TNFα, modulating inflammatory pathways while minimizing systemic side effects 5.
Another promising development includes ADCs targeting CD6, which selectively eliminates pathogenic T cells implicated in autoimmune disorders like lupus and graft-versus-host disease 6. Similarly, CD45-targeted ADCs aim to reset the immune system by eradicating autoreactive immune cells, enabling autologous hematopoietic stem cell transplants in conditions like multiple sclerosis 7. For inflammatory bowel diseases, ADCs are designed to target gut-specific inflammatory markers, ensuring that anti-inflammatory agents are delivered precisely to the intestines 8.
Combating Infectious Diseases
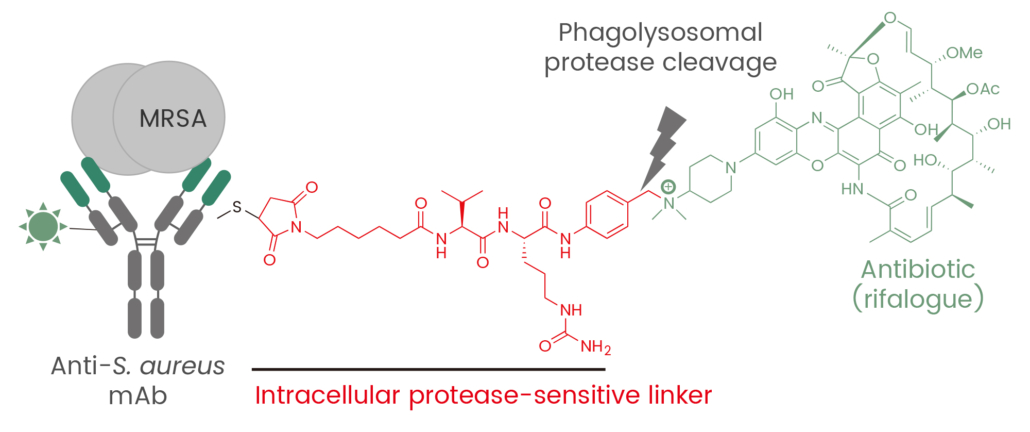
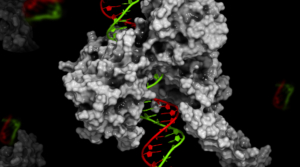
ADCs are emerging as a promising solution to combat the escalating threat of antimicrobial resistance. A notable example is RG7861, an ADC developed to target S. aureus, a bacterium associated with severe infections. RG7861 uses a monoclonal antibody that binds to wall teichoic acid—a key bacterial structure—conjugated with a rifamycin analog. This design allows RG7861 to penetrate bacterial defenses and deliver its therapeutic payload effectively. Clinical trials have shown promising results, highlighting its potential as a next-generation antibiotic 9,10.
Researchers are also developing ADCs for treating HIV-1 by linking monoclonal antibodies that target viral proteins such as gp120 and gp41 to small molecule antivirals, aiming to block viral entry and replication 11.
Figure 2.Antibody-antibiotic conjugate for treatment of S. aureus infections
DOI: 10.1038/nature16057
Neurological Disorders
For Alzheimer’s disease, amyloid-beta plaques or tau protein aggregates are known to contribute to cognitive decline. ADCs designed to target these proteins could slow disease progression12. In multiple sclerosis and other autoimmune neurological conditions, ADCs aim to modulate pathogenic immune cells or inflammatory pathways within the central nervous system. Emerging research is also exploring ADC applications for conditions such as Parkinson’s and Huntington’s disease, where targeted drug delivery could improve patient outcomes. Although still experimental, the potential to address drug delivery challenges in the brain marks a significant advancement in the treatment of neurological disorders 2,13.
Addressing Metabolic Disorders
Metabolic disorders, including atherosclerosis, diabetes, and obesity, are increasingly being targeted with ADC technology 2,14,15. Unlike traditional ADCs, metabolic applications often involve non-cytotoxic payloads designed to modulate biochemical pathways with precision. For example, ADCs targeting lipid metabolism are being developed to address atherosclerosis. An LXR agonist–ADC, for instance, delivers its payload to specific lipid pathways, helping to modulate cholesterol levels and reduce plaque formation15,16.
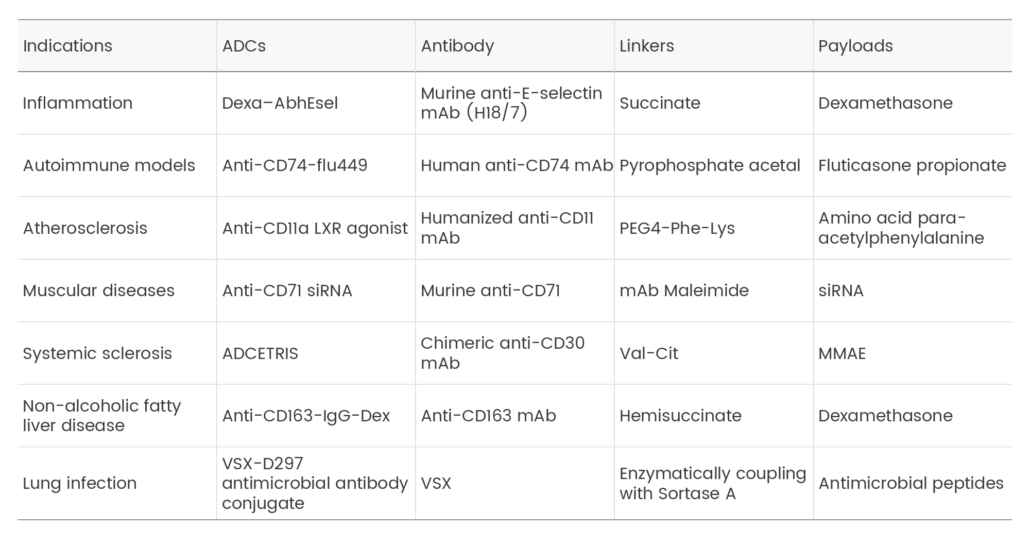
Table 1. Selected ADCs that have been tested for indications other than oncology.
Conclusion and Future Outlook
ADCs have evolved from being primarily oncology-focused into versatile tools with transformative potential across various therapeutic areas. Their ability to combine precision targeting with potent therapies offers a pathway to address complex diseases, ranging from autoimmune disorders to metabolic and neurological conditions, as well as antimicrobial resistance. Looking ahead, advancements in linker technology, antibody design, and payload engineering will likely expand the applications of ADCs even further. The integration of ADCs with emerging fields like gene therapy, nanotechnology, and personalized medicine could unlock new possibilities. As clinical research continues to validate their efficacy and safety, ADCs are poised to become central players in modern therapeutics, addressing unmet medical needs and improving patient outcomes.1–4,17–21
Sino Biological’s Efforts on New Trends of ADC Development
Sino Biological is leading the way in advancing ADC research with a broad portfolio of products and services. Our end-to-end ADC development solutions support every stage of the process—from early discovery through clinical development—ensuring seamless support for our clients. With a strong focus on recombinant protein production, we offer a diverse collection of high-quality ADC target proteins available in multiple species and formats. Our catalog includes well-known targets such as HER-2, TROP-2, Nectin-4, EGFR, CD19, and BCMA, along with emerging targets like EphA3, GFRA1, and CLEC7A.
We also provide comprehensive solutions for autoimmune diseases, offering a wide range of target proteins for nearly 50 diseases. These reagents support the applications of innovative ADCs in autoimmune diseases.
References:
- Tsuchikama, K., Anami, Y., Ha, S. Y. Y. & Yamazaki, C. M. Exploring the next generation of antibody–drug conjugates. Nature Reviews Clinical Oncology vol. 21 203–223 Preprint at https://doi.org/10.1038/s41571-023-00850-2 (2024).
- Pal, L. B., Bule, P., Khan, W. & Chella, N. An Overview of the Development and Preclinical Evaluation of Antibody–Drug Conjugates for Non-Oncological Applications. Pharmaceutics vol. 15 Preprint at https://doi.org/10.3390/pharmaceutics15071807 (2023).
- Conilh, L., Sadilkova, L., Viricel, W. & Dumontet, C. Payload diversification: a key step in the development of antibody–drug conjugates. Journal of Hematology and Oncology vol. 16 Preprint at https://doi.org/10.1186/s13045-022-01397-y (2023).
- Dumontet, C., Reichert, J. M., Senter, P. D., Lambert, J. M. & Beck, A. Antibody–drug conjugates come of age in oncology. Nature Reviews Drug Discovery vol. 22 641–661 Preprint at https://doi.org/10.1038/s41573-023-00709-2 (2023).
- McPherson, M. J. et al. An anti–TNF–glucocorticoid receptor modulator antibody-drug conjugate is efficacious against immune-mediated inflammatory diseases. Sci Transl Med 16, 8936 (2024).
- Zhang, L. et al. A CD6-targeted antibody-drug conjugate as a potential therapy for T cell-mediated disorders. (2023) doi:https://doi.org/10.1172/jci.
- Brandish, P. E. et al. Development of Anti-CD74 Antibody-Drug Conjugates to Target Glucocorticoids to Immune Cells. Bioconjug Chem 29, 2357–2369 (2018).
- Rimola, J. et al. ADC Values for Detecting Bowel Inflammation and Biologic Therapy Response in Patients With Crohn Disease: A Post Hoc Prospective Trial Analysis. American Journal of Roentgenology 222, (2024).
- Lehar, S. M. et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 527, 323–328 (2015).
- DSTA4637S (Anti-S. aureus TAC; RG7861) » ADC Review. https://www.adcreview.com/drugmap/dsta4637s/.
- Umotoy, J. C. & de Taeye, S. W. Antibody Conjugates for Targeted Therapy Against HIV-1 as an Emerging Tool for HIV-1 Cure. Frontiers in Immunology vol. 12 Preprint at https://doi.org/10.3389/fimmu.2021.708806 (2021).
- Punyakoti, P. et al. Postulating the possible cellular signalling mechanisms of antibody drug conjugates in Alzheimer’s disease. Cell Signal 102, 110539 (2023).
- Exploring the potential of ADCs beyond oncology. https://www.drugtargetreview.com/article/151932/exploring-the-potential-of-adcs-beyond-oncology/.
- Xue, D., Liu, P., Chen, W., Zhang, C. & Zhang, L. An anti-CD103 antibody-drug conjugate prolongs the survival of pancreatic islet allografts in mice. Cell Death Dis 10, (2019).
- Liu, Y. Bin et al. A sterol analog inhibits hedgehog pathway by blocking cholesterylation of smoothened. Cell Chem Biol 31, 1264-1276.e7 (2024).
- Ayyappan, J. P., Paul, A. & Goo, Y. H. Lipid droplet-associated proteins in atherosclerosis (Review). Molecular Medicine Reports vol. 13 4527–4534 Preprint at https://doi.org/10.3892/mmr.2016.5099 (2016).
- Valsasina, B., Orsini, P., Terenghi, C. & Ocana, A. Present Scenario and Future Landscape of Payloads for ADCs: Focus on DNA-Interacting Agents. Pharmaceuticals vol. 17 Preprint at https://doi.org/10.3390/ph17101338 (2024).
- Ruiz, R. & Kirk, A. D. Long-Term Toxicity of Immunosuppressive Therapy. in Transplantation of the Liver 1354–1363 (Elsevier, 2015). doi:10.1016/B978-1-4557-0268-8.00097-X.
- Gu, Y., Wang, Z. & Wang, Y. Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharmaceutica Sinica B vol. 14 1965–1986 Preprint at https://doi.org/10.1016/j.apsb.2024.01.009 (2024).
- Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nature Reviews Drug Discovery vol. 16 315–337 Preprint at https://doi.org/10.1038/nrd.2016.268 (2017).
- Carter, P. J. & Lazar, G. A. Next generation antibody drugs: Pursuit of the ‘high-hanging fruit’. Nature Reviews Drug Discovery vol. 17 197–223 Preprint at https://doi.org/10.1038/nrd.2017.227 (2018).



 Sino Biological
Sino Biological