UK retains Europe’s top biotech VC spot despite 2025 slowdown
UK biotech is heading into 2026 with a growing sense of momentum, even as the sector continues to navigate one of the most selective investment climates in years.
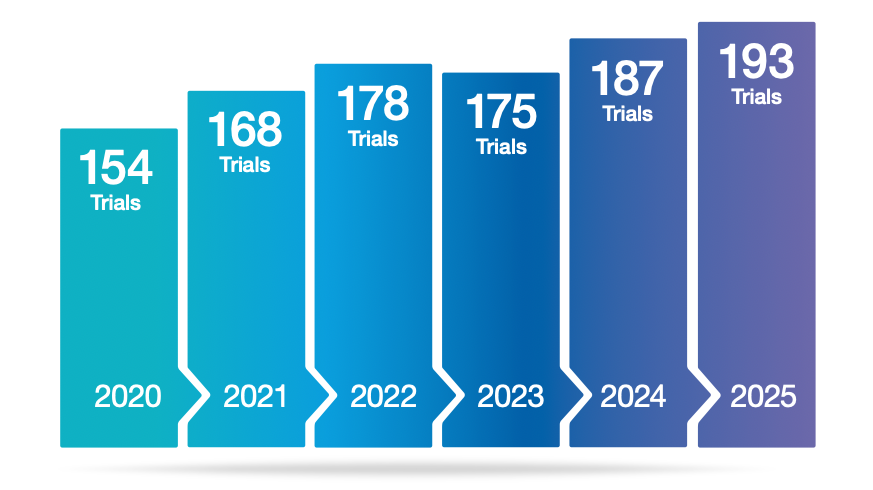
New data from the BioIndustry Association (BIA) shows that while venture capital investment in UK biotech fell in 2025, deal activity accelerated toward the end of the year, a sign that investor appetite may be widening again. At the same time, the UK’s advanced therapy ecosystem continues to expand, with the number of ongoing advanced therapy medicinal product (ATMP) clinical trials rising to 193 in 2025, supported largely by commercial sponsors and dominated by gene therapy development.
Taken together, the figures point to a sector that remains under pressure but increasingly defined by resilience, strategic capital allocation, and continued clinical progress.
A selective year for funding, but a stronger finish
According to the BIA’s UK biotech financing 2025 report, UK biotech raised £1.9 billion in equity financing in 2025, a sharp decline compared with 2024. Venture capital accounted for £1.79 billion across 58 deals, representing a 13.2% year-on-year decrease, a downturn driven by lower deal volume and a market that remained highly selective.
But while much of the year was characterised by investor caution, the BIA report highlights a stronger end to 2025: Q4 recorded 22 completed deals, the highest quarterly deal count of the year. That uptick suggests that, even in a challenging macro environment, investors were still willing to back UK science and that confidence improved as the year progressed.
The report also points to a financing landscape increasingly shaped by concentration: two large Q1 transactions (Isomorphic Labs raised £448.9 million and Verdiva Bio £327.2 million) represented a substantial share of total capital raised, lifting the average deal size to around £30 million, even as mid-sized scale-up financings remained constrained.
UK remains Europe’s biggest biotech venture market
Despite the overall decline in venture funding, the UK retained its position as Europe’s leading national biotech market, representing 30% of all European venture financing in 2025.
That figure matters because it reinforces a long-standing narrative: while UK biotech often faces challenges at the scale-up stage — particularly around later-stage funding and IPO liquidity — it continues to attract global investor attention and remains one of the most important markets for European biotech formation and growth.
The investor mix is also telling. The BIA report highlights how international capital remains dominant in later-stage rounds, underlining both the strength of UK opportunities and the ongoing reliance on overseas funding for scaling beyond early development.
IPOs remain absent, but M&A emerges as a key route to liquidity
Public markets remain the clearest weak spot. In 2025, no UK biotech companies completed an IPO, marking a third consecutive year without new listings. Follow-on financing also contracted sharply, with listed companies raising a relatively small amount compared with the prior year.
In contrast, mergers and acquisitions provided one of the strongest signals of international confidence in UK biotech. The BIA report points to several high-value transactions, led by MSD’s £7.5 billion acquisition of Verona Pharma, alongside other strategic acquisitions by major pharma players.
In a market where the IPO window remains restricted, dealmaking has increasingly become the practical route to liquidity and an important mechanism for recycling capital back into earlier-stage innovation.
Advanced therapies keep moving forward: 193 ongoing UK ATMP trials in 2025
While funding volatility continues to shape biotech strategy, the UK’s clinical activity in advanced therapies remains robust.
New data presented in the Cell and Gene Therapy Catapult’s UK 2025 ATMP Clinical Trials Database shows the number of ongoing advanced therapy clinical trials in the UK reached 193 in 2025. Importantly, 56% of these trials were early-stage, indicating a pipeline that is still heavily driven by clinical translation and proof-of-concept work rather than late-stage programmes alone.
The trial dataset also highlights the central role of industry: around 80% of ATMP clinical trials were commercially sponsored, reflecting the scale, operational complexity, and capital intensity required to run cell and gene therapy studies.
At a time when many biotech companies globally are being forced to cut costs and prioritise fewer programmes, the continuing flow of early-stage ATMP trials suggests the UK retains meaningful execution capacity, including specialist trial sites, delivery infrastructure, and clinical expertise that can support complex modalities.
Gene therapy dominates, with oncology still the largest indication
The Catapult database also confirms that gene therapy continues to dominate the UK ATMP landscape, with more than 80% of trials falling into this category and an even split between ex vivo and in vivo approaches.
Oncology remains the leading therapeutic focus, accounting for 35% of trials. But the dataset points to growth beyond cancer, including increasing activity in disease areas such as inflammation and immune system conditions, reinforcing how advanced therapies are expanding into broader clinical categories.
Policy reforms and reimbursement signals add to optimism for 2026
Industry leaders say the UK is increasingly well-positioned to benefit from both the health impact and economic potential of advanced therapy adoption, provided the system can keep pace with the demands of these treatments.
Matthew Durdy, Chief Executive of the Cell and Gene Therapy Catapult, pointed to the UK’s regulatory support and evolving reimbursement outlook as reasons for confidence.
“With a supportive regulator and recent updates such as the increase to the NICE thresholds, the UK is well-positioned to benefit from the health and economic benefits of advanced therapy clinical trials,” Durdy said. “These trials are vital to ensuring the healthcare system is ready to adopt innovative therapies more widely, as they help build the skills, infrastructure, and clinical environment needed to deliver these therapies safely and efficiently.”
Meanwhile, the BIA financing report argues that the macro-environment heading into 2026 could strengthen the sector further, including improving sentiment, positive signals from US markets, and policy initiatives designed to unlock new pools of domestic capital.
A sector that is maturing, even as it remains constrained
If 2025 was defined by selective financing, the data suggests it was also a year of strategic maturation for UK biotech.
Venture investors narrowed their focus, funding became more concentrated, and public exits remained limited. Yet clinical progress continued — particularly in advanced therapies — and the UK retained its position as Europe’s top national biotech venture market.
The emerging picture is of an ecosystem that remains highly competitive globally, capable of producing science and programmes that attract international interest, while still working through structural challenges around scale-up funding and public market access.
For 2026, the mandate is clear: if the UK can convert improving investor sentiment and policy ambition into sustained capital deployment, and if the clinical system continues building the infrastructure required for next-generation therapies, it may be able to turn resilience into acceleration.




 gov.uk
gov.uk