
Ten drug recommendations by EMA
EMA’s human medicines committee (CHMP) recommended ten medicines for approval at its June 2024 meeting.
The committee recommended end of June granting a marketing authorisation for Balversa (erdafitinib), for the treatment of adult patients with unresectable or metastatic urothelial carcinoma, a cancer of the bladder and urinary system.
The CHMP adopted a positive opinion for Eurneffy (epinephrine), the first emergency treatment against allergic reactions that is administered as a nasal spray, not as an injection. See more details in the news announcement in the grid below.
mResvia (Respiratory Syncytial Virus (RSV) mRNA vaccine) received a positive opinion from the CHMP for prevention in adults 60 years of age and older of lower respiratory tract disease and acute respiratory disease caused by respiratory syncytial virus, a common respiratory virus that usually causes mild, cold-like symptoms but can lead to serious consequences in older adults. This is the first mRNA vaccine targeting a different pathogen than SARS-CoV-2 that receives a positive opinion from the CHMP.
The committee recommended granting a conditional marketing authorisation for Ordspono* (odronextamab), for the treatment of follicular lymphoma and diffuse large B-cell lymphoma, two types of blood cancer that affect the immune system.
Piasky (crovalimab) received a positive opinion from the CHMP for the treatment of paroxysmal nocturnal haemoglobinuria, a rare genetic disorder that causes the premature breakdown of red blood cells by the immune system and is potentially life-threatening.
The CHMP gave a positive opinion for Tauvid (flortaucipir (18F)), for positron emission tomography (PET) imaging of the brain in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease.
The CHMP recommended granting a marketing authorisation for Winrevair* (sotatercept), to treat adult patients with pulmonary arterial hypertension, a rare, long-term, debilitating and life-threatening condition in which patients have abnormally high blood pressure in the arteries in the lungs. This medicine was supported through EMA’s Priority Medicines (PRIME) scheme, which provides early and enhanced scientific and regulatory support for promising medicines with a potential to address unmet medical needs. See more details in the news announcement in the grid below.
The committee recommended granting a marketing authorisation for Steqeyma (ustekinumab), a biosimilar medicine for the treatment of adult patients with moderately-to severely-active Crohn’s disease, plaque psoriasis, paediatric plaque psoriasis and psoriatic arthritis.
The committee also adopted positive opinions for two generic medicines:
- Enzalutamide Viatris (enzalutamide) for the treatment of prostate cancer.
- Nilotinib Accord (nilotinib) for the treatment of Philadelphia chromosome positive chronic myelogenous leukaemia.



 Romi - adobe stock.com
Romi - adobe stock.com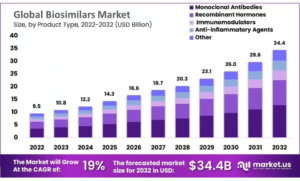 market.us
market.us