MYR Pharma completes chronic hepatitis delta POC trial
German MYR Pharma has completed a proof of concept Phase IIb trial with its first-in-class hepatitis D entry inhibitor Myrcludex B, which is developed under the European Medicines Agency's PRIME scheme.
In February, the company had announced that Myrcludex B + interferon treatment was well tolerated by 60 patients with chronic hepatitis D during the 48-week active treatment phase. The 47-amino acid synthetic peptide drug targets the recently identified HBV receptor hepatocyte surface protein NTCP. By blocking NTCP, Myrcludex B prevents the infection of the healthy cells by the hepatitis B virus (HBV) and co-infecting hepatitis D virus (HDV) and thus viral spreading within the liver.
Currently, chronic hepatitis Delta (HDV), the most severe form of viral hepatitis, has no satisfactory treatment option. The company said, detailed results of both the active treatment phase and the 24-week follow-up phase will be presented at the General Session of the upcoming International EASL Liver Congress in Paris, France on April 12, 2018.
Myrcludex B has received Orphan Designation for treatment of HDV infection from the EMA and FDA, and PRIME scheme eligibility from the EMA. MYR Pharma sees also potential to treat a range of inflammatory and metabolic diseases with the drug candidate originally developed by Professor Stephan Urban at University of Heidelberg, Germany. Because Myrcludex B’s target NTCP is involved in the bile acid transport cycle, MYR Pharma believes, the linear peptide could also be used for the treatment of various metabolic and inflammatory diseases.
MYR Pharma is one of the current nine portfolio company’s of the Russian US$100m Maxwell Biotech Venture Fund (MBVF) and of the German High Tech Gründerfonds. The company conducts its clinical trials in Russia.


 T. Rowe Price
T. Rowe Price Sandoz
Sandoz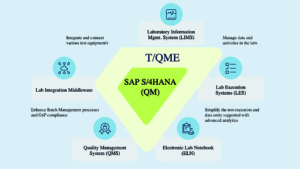 Tenthpin Solutions AG
Tenthpin Solutions AG