Lindis Biotech selects Celonic Group for commercial manufacturing of catumaxomab
LINDIS Biotech GmbH has selected Swiss Celonic Group to manufacture its recently EU-approved malignant ascites treatment catumaxomab for commercial supply.
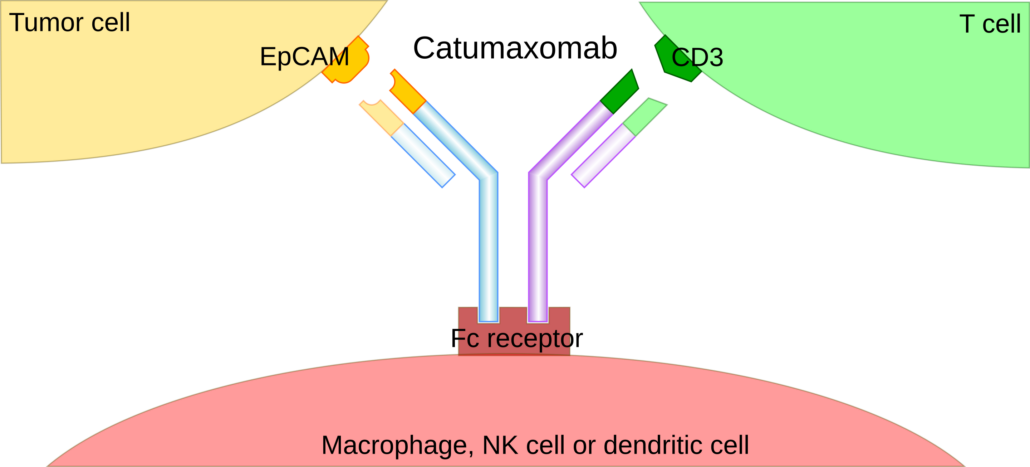
Celonic Group has inked a long-term agreement with LINDIS Biotech GmbH to manufacture its EpCAM/CD3 T cell engager catumaxomab at commercial scale in its Heidelberg facilities. Most recently, LINDIS Biotech has received EU re-approval for catumaxomab, making the the only drug approved for the specific and cancer-directed treatment of malignant ascites. Under a licence agreement, LINDIS has granted Pharmanovia exclusive rights to launch the trifunctional antibody catumaxomab, which was re-approved by the EU Commission in mid-February, and to manage the product launch throughout Europe.
Under the manufacturing agreement with the Swiss CDMO , Celonic Group will manufacture catumaxomab for commercial supply in its state-of-the-art GMP manufacturing facility in Heidelberg, Germany. Malignant ascites is an abnormal accumulation of fluid in the peritoneal cavity that commonly arises from advanced-stage cancers.
Dr. Horst Lindhofer, CEO of LINDIS Biotech, commented: “Partnering with Celonic Group is a vital part of our mission to bring catumaxomab to patients suffering from malignant ascites and fill this unmet clinical need. With Celonic’s proven manufacturing capabilities and commitment to quality, we are confident in their ability to ensure a reliable and high-quality supply of our innovative therapy.”
Although catumaxomab is described by the EU Commission as a first-in-class therapeutic, it was the world’s first approved bi-specific antibody back in 2009. Due to a lack of success, the developer Trion Pharma GmbH and its marketing partner Fresenius Biotech withdrew the trifunctional antibody from the market in 2017. Following the insolvency of Trion Pharma, its founder Horst Lindhofer bought back the patents from the insolvency estate and founded Lindis Biotech. This company succeeded in obtaining marketing authorisation again without having to generate new clinical data.
Samanta Cimitan PhD, CEO of Celonic Group, said: “We are honored to collaborate with LINDIS Biotech on the production of catumaxomab. This partnership underscores our dedication to supporting the development and commercialization of groundbreaking biologics that address unmet medical needs.”


 stock.adobe.photo.com/Popelniushka
stock.adobe.photo.com/Popelniushka  Boehringer Ingelheim
Boehringer Ingelheim Adobe stock photos - nadia
Adobe stock photos - nadia