French EG 427 bags €27m in Series B financing
French targeted DNA gene therapy specialist EG427 has closed a €27m Series B financing co-led by VC investor Andera Partners and BPI France to initiate Phase I testing of a Herpes Simplex Virus 1 (nrHSV-1) vector that specifically targets type C neurons to suppress dysfunctions of the bladder in people with spinal injury.
Besides lead investors Andera Partners and Bpifrance, SCI Ventures, several spinal cord injury foundations and existing investors participated in the €27m Series B round of EG 427 SA. The Paris-headquartered company said the funds will be used to finance Phase Ib/IIa trials of its lead candidate EG110A conducted at four study sites in the USA. A DNA-encoded study drug that blocks type C neurons near the spinal cord will be administered to adult patients with neurogenic detrusor overactivity (NDO) who had a spinal cord injury at least 12 months ago in order to reduce the number and time of bladder leakages (uninary incontinence). Safety and hints to efficacy will be followed initially for 12 months. After that, reponders will be asked to enter a 5-year follow-up observational period.
EG110A is a genetic medicine utilizing a non-replicating HSV-1 vector, which has been designed to selectively silence the signals of key bladder sensory neurons responsible for the bladder muscle overactivity, whilst preserving bladder voiding function. NDO is a common urinary bladder dysfunction caused by SCI and other neurodegenerative diseases, such as multiple sclerosis or Parkinson’s disease.
Results of preclinical tests seem promising. In a non-clinical pharmacology study in an OAB model, EG110A showed similar efficacy and a strong safety profile compared to botulinum toxin A, notably without the increase in post-void residual urine volume, a known adverse reaction in NDO and OAB with botulinum toxin A. In a long-term non-clinical kinetic study, EG110A demonstrated persistent transgene expression in the dorsal root ganglia in animals of at least six months. It is the first human study of such a vector, targeting sensory neuron-based diseases.
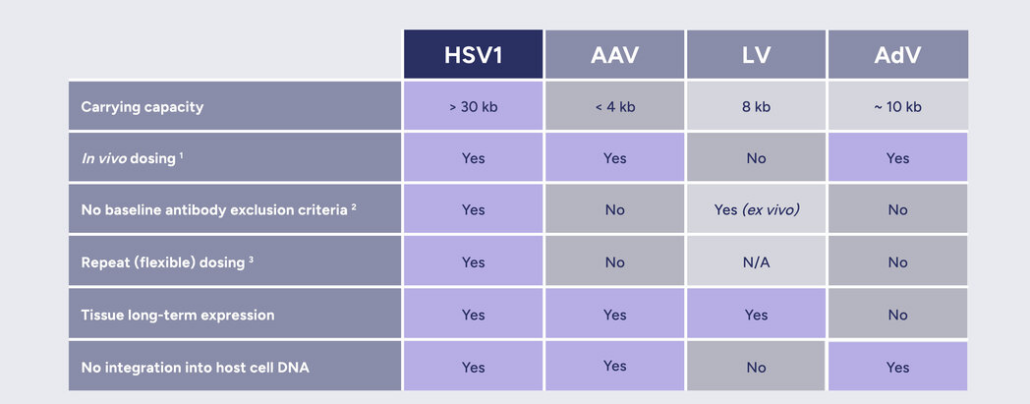
In addition to the first-in-man study, EG 427 plans to use some money to support the company’s earlier-stage pipeline based on its proprietary HERMES vector technology platform, that delivers large DNA pieces to specific neurons of the peripheral and autonous nerve system with help of a non-replicating HSV-1 vector that „promises affordable production costs compared to mostly used AAV-based vectors,“ according to Benoît Barteau, Investment Director at Bpifrance‘s InnoBio funds.


 FDA
FDA gov.uk
gov.uk uniqure
uniqure