Citryll BV raises €85m in Series B financing
Dutch inflammation specialist Citryll BV has raised €85m to push clininal testing of its lead candidate, CIT-013, a bifunctional antibody that prevents the release of neutrophil extracellular traps (NETs) from neutrophil granulocytes into the cytoplasm, thus blocking inflammatory and autoimmune diseases at its very roots.
Following a €18.5m Series A round in 2020, Citryll BV (Oss, The Netherlands) has raised €85m in an oversubscribed Series B round. The funding round was co-led by Johnson & Johnson Innovation, Forbion, and Novartis Venture Fund, with the participation of Pureos Bioventures, alongside existing investors BioGeneration Ventures, Series A lead Seventure Partners, BOM, Curie Capital, and Citryll’s founders from ModiQuest BV, originator of the tACPA patents, Helmuth van Es, former CEO Citryll, and Renato Chirivi, former CSO, now CTO Citryll and co-inventor of tACPA.
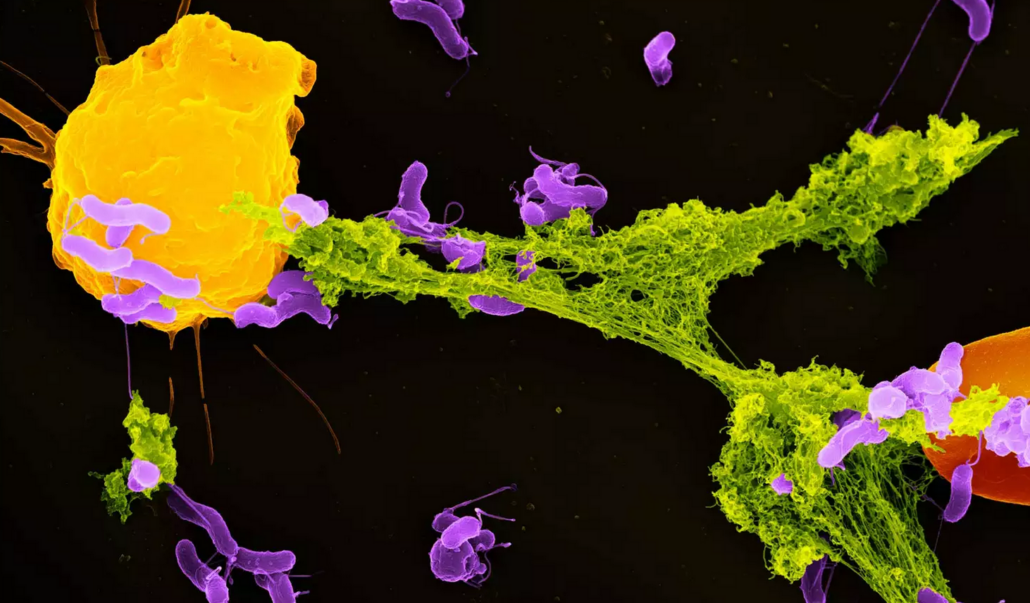
The funding will enable next steps for the clinical development of CIT-013, a first-in-class monoclonal antibody that targets Neutrophil Extracellular Traps (NETs) through binding to Histone 2A and Histone 4. CIT-013 does not broadly target inflammation or acquired immunity, instead it extinguishes the source of autoantigens, such as NET-derived toxic, prothrombotic and proinflammatory components and citrullinated histone proteins. NETs, which were discovered by Volker Brinkmann at MPI for Infection Biology in Berlin, are mesh-like structures composed of DNA, histones, and antimicrobial proteins, released by neutrophils to trap and degrade pathogens. Excessive NET formation can contribute to tissue damage and chronic inflammation in various immune-mediated inflammatory disorders, including sepsis, acute respiratory distress syndrome (ARDS), acute lung injury (ALI), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), vasculitis, antiphospholipid syndrome (APS), and asthma.
Citryll recently completed its first-in-human Phase I studies including successful repeat dosing of eight rheumatoid arthritis (RA) patients. Phase IIa trials are planned in both RA and hidradenitis suppurativa (HS), intended to establish CIT-013’s unique dual mechanism of action, which supports the clearance of existing NETs and inhibits the formation of new NETs. CIT-013 is highly selective for its epitope, minimising off-target effects, and it does not enter cells, preserving normal intracellular functions. While initially focusing on RA and HS, Citryll’s NET-targeting approach has potential applications across a wide range of immune-mediated inflammatory diseases.
Following this Series B round, Geert-Jan Mulder, Managing Partner at Forbion, Florian Muellershausen, Managing Director at Novartis Venture Fund, and a representative of JJDC will join Citryll’s Board as non-executive directors.




