Bayer inks US$1.55bn cancer deal with Loxo Oncology
German Bayer AG has acquired ex-US commercialisation rights to Loxo Oncology Incs Phase II lead larotrectinib (LOXO-101), which targets TRK (tropomyosin receptor kinase) fusions in 17 cancer types, and LOXO-195, a Phase I/II compound designed to prevent acquired resistance against larotrectinib.
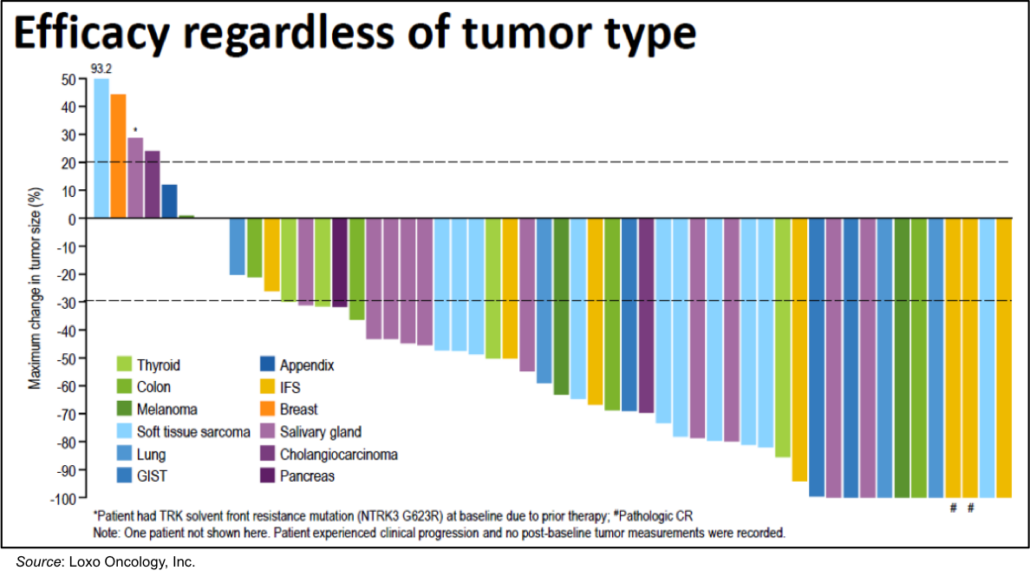
Bayer paid US$400m upfront for ex-US commercialition rights and promised as much as $1.15bn milestone payments in case of success as well as a 50/50 split of any future profits from the drugs in the US. This summer, Connecticute-based Loxo presented data at ASCO 2017 from a basket trial, which enroled 50 patients with 17 different cancer types, suggesting treatment with larotrectinib led to a 76% objective response rate.
The first-in-class drug candidate, which was acquired from Array BioPharma Inc. in 2013, blocks TrkA (neurotrophic tyrosine kinase receptor 1, NTRK1), TrkB (NTRK2) and TrkC (NTRK3) and received FDA breakthrough therapy designation to treat end-stage cancer patients expressing NTRK-fusions. LOXO-195 is a follow-up next-generation Trk blocker, which could be given when patients become resistant to larotrectinib treatment.
Under the contract, Loxo will lead clinical development globally and will be responsible for NDA submission in the US (expected for H1/2018). Bayer will pay 50% of the development costs. Bayer has also aquired the global commercialisation rights except for the US where the partners will share future profits.




 adobe stock - peterschreiber.media
adobe stock - peterschreiber.media