Aplagon secures €7m for APAC development
Helsinki-based Aplagon has secured €7m in financing to advance its therapeutic APAC into Phase 2a clinical trials.
APAC, a potential first-in-class therapeutic for thrombo-inflammatory diseases, is a heparin proteoglycan mimetic designed to target arterial injury sites, providing antithrombotic and anti-inflammatory action. The investment will support Aplagon’s clinical development strategy, specifically initiating a Phase 2a clinical trial for peripheral arterial occlusive disease/chronic limb threatening ischemia (PAOD/CLTI) and completing three ongoing clinical trials: a Phase I Arteriovenous Fistula maturation failure trial, a Phase I intravenous trial in healthy participants, and a PET-imaging trial with 89zirconium-labeled APAC. “Our innovative approach, using a heparin proteoglycan mimetic with targeting ability and retention on the vascular injury sites, has potential applications across a broad range of serious vascular diseases. We are looking forward to announcing clinical results from these trials in 2025 and 2026,” commented Aki Prihti, CEO at Aplagon.
The funding round was led by Fåhraeus Startup and Growth AB (FSG) and the European Innovation Council (EIC) Fund, with additional support from Finnish investors including the Jenny and Antti Wihuri Foundation, Innovestor and Gösta Serlachius Fine Arts Foundation. “Thrombo-inflammation is a key underlying driver for many cardiovascular diseases”, said Johanna Asklin, General Partner at FSG, who will join Aplagon‘s Board. “At FSG we look for revolutionary innovations, and we found precisely that with Aplagon’s APAC approach to treat this clear unmet medical need.”


 Rosa Therapeutics Inc
Rosa Therapeutics Inc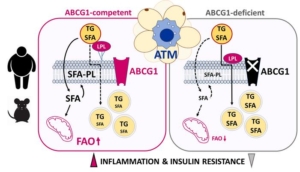 Wilfried Le Goff (INSERM, Sorbonne Université)
Wilfried Le Goff (INSERM, Sorbonne Université) Catalym
Catalym