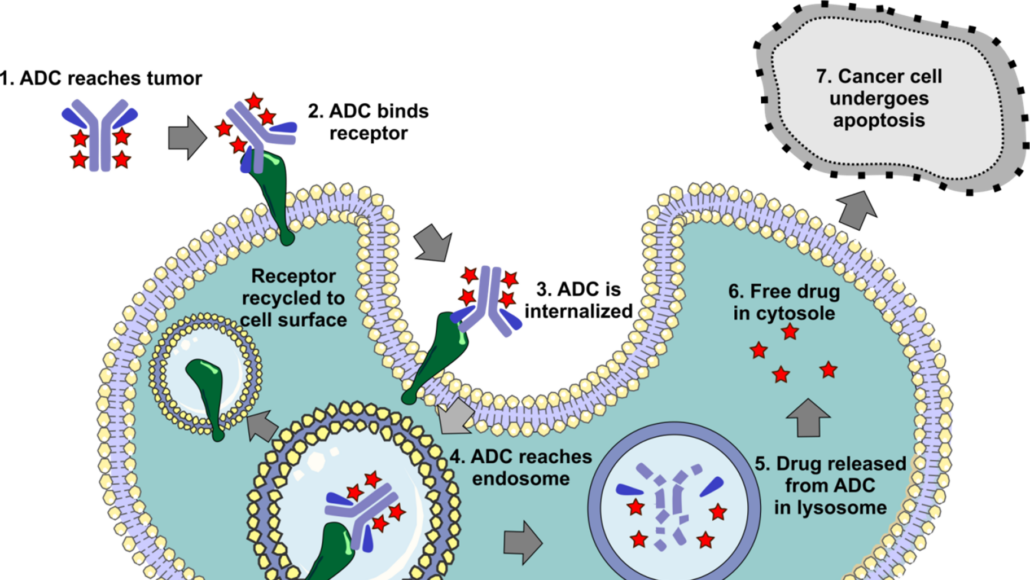
Adcendo broadens ADC pipeline in US$1bn biobucks deal
Danish ADC maker Adcendo ApS has licenced the rights to commercialise Multitude Therapeutics Inc's first-in-class ADCE-T02 globally except the Greater China region.
Copenhagen-based Adcendo ApS has aquired the rights to market the first-in-class tissue factor-targeting antibody drug conjugate ADCE-T02 from Chinese Multitude Therapeutics Inc. Tissue factor (TF) is a transmembrane protein that plays a crucial role in thrombosis. TF expression is elevated in various types of cancer, including colorectal cancer (CRC), cervical and pancreatic cancer, and is associated with more aggressive disease and poor prognosis.
Under the agreement, Adcendo will obtain the exclusive development and commercialisation rights for the asset globally, except for the Greater China region (Mainland China, Hong Kong Special Administrative Region, Macao Special Administrative Region, and Taiwan) where Multitude sees its market. According to the contract, Multitude can receive upfront and milestone payments totalling US$1bn, as well as single-digit to low double-digit tiered royalties on future product sales.
Tissue factor (Factor III) is a clinically validated ADC target that is highly expressed in indications such as non-small cell lung cancer (NSCLC), colorectal cancer (CRC), cervical, oesophageal , head and neck , bladder and certain gastrointestinal cancers, but its expression is low in normal tissues.
ADCE-T02 is an anti-TF ADC, and the first ADC with a topoisomerase I inhibitor-based linker/payload, to enter into clinical development in Australia, US and Europe. The start of the Phase I study in Australia is expected in Q4 2024. Its antibody design minimises the impact on the coagulation pathway, while the T1000-exatecan linker-payload technology platform has been preclinically demonstrated to amplify the bystander effect, improve linker stability, and has the potential to overcome potential resistance mechanisms.
Adcendo ApS in 2024 completed a Series A extension financing round, increasing the total funds raised to €98m to advance, broaden, and accelerate the development of its first-in-class ADC pipeline assets. Investors include Novo Holdings, Ysios Capital, Pontifax Venture Capital, RA Capital Management, HealthCap, Gilde Healthcare and Dawn Biopharma, a platform controlled by KKR.
Multitude Therapeutics Inc (Shanghai, China) is a clinical-stage company focused on the development of ADC drugs. Multitude Therapeutics has two technology platforms: MabArray™— an antibody platform for discovering novel cell surface tumour targets to construct first-in-class targets, and T1000 — a new linker-payload technology for developing ADCs, which allows ADCs prepared with this platform to achieve a better balance of the bystander effect, efficacy, and safety. According to the Shanghai-headquartered company, the combination of MabArray™ and T1000 generates synergistic effects, enabling Multitude Therapeutics to build an ADC “atlas” that is expected to treat malignant tumors and achieve higher and more durable responses.


 Araris Biotech AG
Araris Biotech AG Lonza Group
Lonza Group International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s
International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s