Richter-Helm, a leading steadily growing Germany-based contract development and manufacturing organization (CDMO), offers excellent services in the continuously growing market of pharmaceutical biotechnology. Two additional bioreactors with capacities of 300 litres and 1,500 litres for microbial production will be added. This includes also the establishment of mid- and downstream processing and supporting utilities on a total area of about 10,000 m².
ADVERTISEMENT
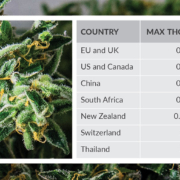
For a plant that has been used for millennia, controls on the supply of psychoactive and non-psychoactive components extracted from cannabis are relatively recent, with global regulations starting in the 20th century with the International Opium Conventions. The current, and often conflicting, regulatory landscape has focused on definitions of hemp and cannabidiol (CBD). Reference materials are critical to defining the % ?9-THC in these products.
Ultra-low freezers, or ULTs, are specialised refrige-rating equipment created to store vaccines, medicines, and samples at ultra-low temperatures. By offering storage temperatures ranging from -20°C to -86°C and energy efficiency, B Medical Systems’ ULTs create an ideal environment for the long-term storage of extremely temperature-sensitive biologicals, making its products perfect for use in laboratory or clinical environments.
The life sciences industry stepped up its pace in the last year to rapidly fill a void in the fight against COVID-19. Solis BioDyne is one of many companies that have experienced a dramatic increase in demand for its solutions during the pandemic especially for products used in COVID-19 diagnostic tests. The company knew it needed to ramp up production. And time was not on their side.
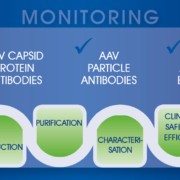
Adeno-associated viruses (AAV) have revolutionised gene therapies as safe and effective gene shuttles. The growing demand for AAV vectors now requires an industrialised process throughout pre-clinical development that involves comprehensive monitoring from production to patient to ensure safety and efficacy of the therapeutic agent and reduce cost of goods.
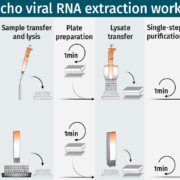
During the global COVID-19 outbreak, BioEcho developed a rapid extraction protocol that reduced the isolation of SARS-CoV-2-RNA to just a few minutes. Currently, BioEcho offers a CE-marked viral RNA/DNA extraction kit and is implementing automated solutions that will allow customers to increase their diagnostics throughput.
No matter whether you’re an end user or an OEM in biophotonics, biomedical optics, and imaging, Coherent has the laser that perfectly matches your needs and your budget.
Innovators at Rentschler Biopharma are continuously developing new processes for biopharmaceutical production to tackle life-threatening diseases. Two of them, Dr Birgit Ehrenberg, Process Manager, and Monika Häußler, Quality Control (QC) Manager, shared insights into their latest projects and how close collaboration with chromatography experts from Tosoh Bioscience helped them develop robust, scalable, and economically viable downstream processes, as well as corresponding reliable bioanalytical methods.
The 11th edition of the European Biotechnology Science & Industry Guide once more offers an interesting cross-section of the European biotech scene.
Exosome Diagnostics develops biofluid-based molecular diagnostic tests for use in personalized medicine and collaborates with pharmaceutical companies to develop companion diagnostics (CDx).