Swiss Ikerian AG’s US-subsidiary, RetinAI U.S. Inc. has announced the first close of an CHF6.73m Series B financing with new and existing investors to advance its AI-based image analysis platform alongside a strategic partnership.
ADVERTISEMENT
German antibody specialist YUMAB GmbH has inked a collaboration agreement with Japanese MOLCURE Inc aimed at using the company’s zero-shot AI technology for antibody discovery.
Dutch Forbion has announced its largest fundraising to date, with Forbion’s Growth Opportunities Fund III raising €1.2bn and Forbion Ventures Fund VII raising €890m.
Lundbeck A/S is set to take over all shares of neurology specialist Longboard Pharmaceuticals Inc worth US$2.6bn to strengthen its pipeline with the Phase III 5-hydroxythyprytamaine 2C receptor super-agonist bexicaserine to treat 11 rare neurological conditions.
German diagnostics company Sphingotec GmbH has licensed its real-time kidney function test to US diagnostics giant Beckman Coulter allowing it to expand its business to the US as announced earlier this month.
AviadoBio Ltd has cashed in €30m upfront and €20m as an equity investment from Astellas Pharma Inc for signing a licence option agreement for its Phase I/II gene therapy programme AVR-01.
Swedish clinical-stage pharmaceutical company Vicore Pharma has secured SEK100m, supported by existing and new investors. The injection of capital will allow the company to scale the clinical development of its portfolio of therapies in respiratory and fibrotic diseases.
Fab’entech, a French biopharma company, has secured a loan worth €20m from the European Investment Bank (EIB) under the HERA Invest programme, aimed at bolstering Europe’s preparedness against biological threats.
Kurma Partners announced a first closing of its new Biofund IV at €140m targeted to raise €250m to finance 16-20 life science companies.
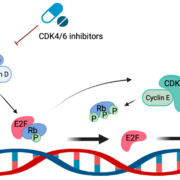
Genentech, the US subsidiary of Swiss pharmaceutical giant Roche AG, has strengthened the company’s breast cancer pipeline by pre-licensing the global commercialisation rights to Regor Therapeutics’ RGT-419B for US$850m.


 Ikerian AG
Ikerian AG Molcure Inc
Molcure Inc Stijnstijl fotografie
Stijnstijl fotografie H.Lundbeck A/S
H.Lundbeck A/S Sphingotec GmbH
Sphingotec GmbH Astellas Pharma Inc
Astellas Pharma Inc AI via Freepik
AI via Freepik EIB
EIB
 https://doi.org/10.3390/ijms241411791
https://doi.org/10.3390/ijms241411791