Roche’s Japanese arm Chugai today returned all rights from a 2016 licence agreement on the RNA polymerase II blocker lurbinectedin to Spanish oncology specialist PharmaMar SA.
ADVERTISEMENT
Selvita spin-out NodThera Ltd raised £28 million (€31.8m) in a Series A financing round led by Sofinnova Partners and 5AM Ventures and co-financed by Epidarex Capital and F-Prime Capital Partners.
Special immune cells that protect the lining of the gut remain in a semi-active state, ready to fend off a pathogen at any moment, thanks to the composition of their mitochondrial membrane, a new study reveals.
A team of Austrian, Hungarian and Canadian researchers has demonstrated that Boehringer Ingelheim’s FDA-approved ERB family kinase blocker afatinib prolonged survival in hard-to-treat K-RAS-mutated lung tumours.
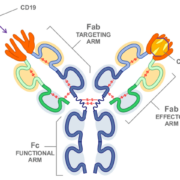
Swiss Novimmune SA has exclusively sold a licence option on its lead product NI-1701 (now renamed TG-1801), a bispecific anti-CD47/anti-CD19 antibody designed to trea B-cell blood cancers, to TG Therapeutics Inc.
For two days, innovation in synthetic and bio-based fine chemicals will be in the focus of experts who meet at Chemspec Europe in Cologne (20-21 June, 2018, Koelnmesse).
Three years ago Roche acquired a majority in shares of Foundation Medicines Inc (FMI), a specialist in cancer panel sequencing, which has build a huge database with cancer mutation profiles. Now Roche paid US2.4bn to complete the take-over.
Researchers at French antibiotics specialist Deinobiotics SAS, a subsidiary of Deinove SA, and German researchers announces the discovery of a new class of resistance breaking antibiotics that kill MRSA or S. pneumoniae effectively.
Following a letter of about 1,000 EPO patent examiners , also patent attorneys have send an open letter to the European Patent Office (EPO), criticising its outgoing president for having established work routines that are set to lower quality of European patents.
Adrenomed AG announced the publication of three scientific papers revealing how its adrenomedullin-targeting Phase II sepsis antibody Adrecizumab reverses vascular hyperpermeability in sepsis.