A team of Scots, Belgian and Spanish researchers claim they have found a target to prevent failure of liver regeneration following intoxination or acute injury.
ADVERTISEMENT
London-headquartered gene therapy Orchard Therapeutics has closed a US-$150m series C financing round to push MAAs and BLAs of three late-stage gene therapies by 2021.
Life Sciences Partners and Andera Partners together with Pfizer Ventures and Novartis Venture Fund have put €85m into the cancer drug development company Artios Pharma Ltd.
German neurogenetics at the Max-Planck-Institute for Experimental Medicine have cured the rare incurable hereditary Charcot-Marie-Tooth disease by simply supplementing the food of mice carrying the disease-causing duplication of the PMP22 gene with lecithin. The phospholipid supplementation enabled Schwann cells deficient in lipid synthesis to restore the insulating myelin sheet around peripheral neurons which is needed to speed up neuronal transmission.
German researchers have found a molecular switch that determines whether mesenchymal fibroblasts differentiate into fat- or bone-forming cells opening the avenue to modulation of the processes.
Researchers at Roche’s US arms Genentech and Foundation Medicine Inc. (FMI) presented a liquid biopsy test that can be used to identify NSCLC patients who respond to Roche’s PD-L1 blocker Tecentriq earlier than to FMI’s tissue based tumour mutation burden (TMB) assay.
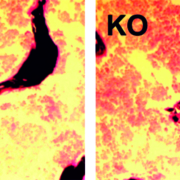
German pharma company and CDMO Boehringer Ingelheim (BI) steps into the lucrative field of gene therapy. Together with the UK Cystic Fibrosis Gene Therapy Consortium (GTC), BI aims at developing replacement therapies compensating for mutations that lead to cystic fibrosis.
An investor consortium led by British Arix Bioscience plc has put £13.4m into the Australian Pharmaxis Ltd, which develops fibrosis inhibitors based on its amine oxidase discovery platform.
As UK’s departure from the EU is fast approaching, stakeholders are preparing for the disruption a hard Brexit might cause. While EMA is scaling back activities, pharma companies are stockpiling meds.
Antiviral drug discovery company ReViral announced the successful completion of a of a US$55m Series B financing. The funding will go toward clinical studies for its respiratory tract infection treatment.


 Boehringer Ingelheim
Boehringer Ingelheim