Lyon-based neurology specialist Theranexus SA has launched a new bioinformatics partnership with systems biology data analysts from Dyliss (Dynamics, Logics and Inference for biological Systems and Sequences).
ADVERTISEMENT
Following the FDA granted AMT-130 fast track designation, uniQure NV announced it will start Phase I/II testing of the Huntington’s Disease gene therapy in Q2/2019.
Sanofi and Alnylam have agreed to end the research and option phase of the companies’ RNAi therapeutics alliance in rare genetic diseases.
Gradientech closes a USD 4.6 million rights issue to progress QuickMIC diagnostic system for ultra-rapid antibiotics susceptibility testing (AST).
Novartis has aquired all outstanding capital stock and paid US$310m upfront to Boston-based IFM Tre Inc., which commercialises three inflammasome blockers designed to treat chronic inflammatory diseases.
The European Medicines Agency has granted conditional approval for bluebird bio NV’s gene therapy Zynteglo (LentiGlobin BB305) to treat the rare disease transfusion-dependent ?-thalassemia at its first meeting in Amsterdam.
Krakow-based Selvita S.A. has announced it will spin out its oncology unit this year resulting in two public companies, a CRO and a cancer therapeutics developer.
The CHF22.5m Series A financing round of the Swiss specialist for autoimmune diseases was led by Sofinnova Partners and New Enterprise Associates together with contributions of existing investors.





 123rf.com/Iculig
123rf.com/Iculig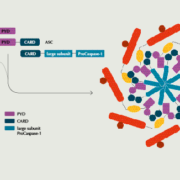
 123rf.com/guniita
123rf.com/guniita