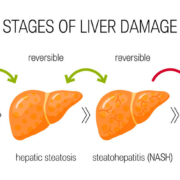
French liver disorder specialist Genfit SA has announced the pricing of its Nasdaq IPO of ADS and European private placement.
ADVERTISEMENT
US-Austrian arenavirus-based cancer and infectious diseases specialist Hookipa has has filed a prospectus for an US$86M Nasdaq IPO.
A research team from Germany and Mexico has successfully targeted uveal melanoma (UM), the most common type of eye cancer.
Pfizer Inc has paid €45m upfront to French gene therapy specalist Vivet Therapeutics in a licence option deal for Vivet’s preclinical Wilson disease candidate VTX-801.
After positive preliminary results of Sotio’s SOV02 trial in ovarian cancer had been presented last year, the final analysis confirmed the survival benefits. The Czech biotech company now plans to initiate a global Phase III study with its autologous cell-based immunotherapy also in this indication.
More than 330 experts joined the 2-day AMR Conference 2019 in Berlin. They called for rapid capital investments and economic incentives in the fight against antimicrobial resistance (AMR).
Researchers at Celldex Therapeutics, Inc and Nerviano Medical Science Srl report elimination of the most common childhood cancer by an ADC in xenografted mice expressing either wild-type or mutated ALK.
Global antibacterial innovation network CARB-X announced during an AMR business conference in Berlin that Germany has joined the CARB-X partnership.