Data from 1,200 heart failure patients demonstrate that high discharge levels of the biomarker bio-ADM® (bioactive Adrenomedullin) indicate residual congestion in heart failure patients.
ADVERTISEMENT
The new company, named Augustine Therapeutics, is a Vlaams Instituut voor Biotechnologie (VIB) and Katholieke Universiteit (KU) Leuven spin-off. Funded with €4.2m from mainly local investors, Augustine’s first priority is finding new therapeutics for patients suffering from Charcot-Marie-Tooth disease, a hereditary motor and sensory neuropathy of the peripheral nervous system.
French Transgene SA said it has terminated its lead programme TG4010 in combination with chemotherapy and checkpoint inhibition.
New research demonstrates that disruption of EGF receptor signalling allows commensal bacteria to invade hairs and cause skin lesions.
Madrid-based RNA specialist Bioncotech Therapeutics has entered into a Phase II clinical trial collaboration with a MSD subsidiary.
Swiss surgeons have significantly improved mechanical removal of blood clots in stroke patients by intra-arterial administration of the thromolytic urokinase.
Sanofi will acquire Synthorx Inc to expand its immuno-oncology pipeline with THOR-707, an optimised IL-2 candidate.
Meatable NV, a specialist for cultivated meat, has raised an additional US$10m in seed funding, bringing the company’s total funding to US$13m.
Médecins Sans Frontières (MSF) called on the GAVI Vaccine Alliance not to pay huge subsidies to Big Pharma companies for pneumococcal vaccine
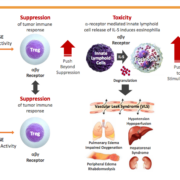
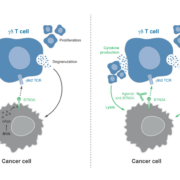
T cell agonist specialist ImCheck Therapeutics will use €48m (US$53m) from a Series B financing to push clincial pipeline of antibodies activating gamma-delta T-cells.