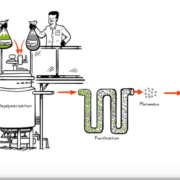
For years, French Carbios SAS has worked to set up a process that effectively depolymerizes PET, one of the most common plastics. Now its process has been published.
ADVERTISEMENT
British researchers have tabled a phylogenetic tree showing different subtypes of SARS-CoV-2 developed in Asia, Europe and the US.
Austrian COVID-19 patients with severe lung dysfunction for the first time have a treatment option within a compassionate use program for Apeptico’s solnatide.
Austrian APEPTICO has got Horizon 2020 money for accelerated development and clinical use of Solnatide for the treatment of COVID-19 patients
The European Medicines Agency EMA has backed eight medicines for approval at its virtual March 2020 meeting.
Scandinavian researchers have created a unified, open-source model providing a genome-wide portrait of human disease metabolism.
Paris-based Novacyt SA has signed a global distribution agreement with Bruker Ltd for its COVID-19 diagnostic test.