As the process determines the product, product characteristics of biological drugs need to be analysed thoroughly right from the beginning of the drug development process. Early understanding of structure-function relationships, aggregation, microheterogenicity, and glycosylation, and more, helps minimise development risks.
ADVERTISEMENT
Germany’s Evotec SE and Boehringer Ingelheim Venture Fund have acquired equal minority stakes in Quantro Therapeutics GmbH, a recently established research-based biotech company based in Vienna. Evotec also entered into a comprehensive partnership with the Austrian start-up, that strives to discover and develop novel therapeutics interfering with disease-causing transcriptional programmes in cancer and other diseases. The Germans will provide hit identification services for Quantro’s proprietary anti-tumour projects.
A biosimilar is a copy version of an approved, original biological medice whose patent protection has expired. The development strategies for biosimilar product and a new biological entity (NBE) differ in many ways.
Viennese APEIRON Biologics AG raised €17.5m to expand ongoing Phase II trials of its
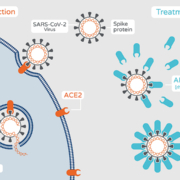
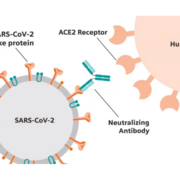
coronavirus-specific drug candidate APN01 in Europe to the US and Russia. The recombinant angiotensin-converting enzyme 2 (rhACE2) mimics the receptor on the human cells that coronaviruses are using to infect these cell. Preclinical and clinical data demonstrate that APN01 is well-tolerated and can stop the overshooting host immune responses that are triggered by virus proliferation.
In a Public England Health study, Siemens Healthineers’ COV2T antibody test was the only assay found to meet both sensitivity and specificity requirements of the MHRA.
After leaving the field of antimicrobials 20 years ago because other areas offered better returns, 23 pharma companies and the Novo Foundation have launched a US$1bn fund to fight antimicrobial resistance (AMR).
Leiden-based coagulant developer VarmX NV has closed a €32m Series B financing to push clinical development of VMX-C001, a venom-based modified recombinant Factor X.
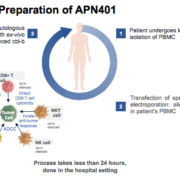
Viennese APEIRON Biologics and MaxCyte Inc have entered into licensing agreement covering the use of Maxcyte’s electroporation platform for APN401 development.
After closing the business merging with blank check company Arya Sciences Acquisition Corp, Immatics Inc has been listed on Nasdaq.
A new insulin formulation allows patients with diabetes to more quickly manage their blood sugar levels during mealtimes.