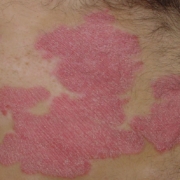
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
ADVERTISEMENT
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
Intravacc has announced positive data for its nasal SARS-CoV-2 vaccine that embeds the viral spike antigen in bacterial Outer Membrane Vesicles (OMV)
In a new statement, the EU regulatory authority EMA postulates that AstraZeneca’s vaccine causes serious deep vein thrombosis.