Biomanufacturing?Implementing high-quality process development that considers GMP manufacturing from the start is critical to reducing costs and timeline disruptions associated with raw material supply chain insecurity, adhering to regulatory compliance, and avoiding process re-design at a later stage.
ADVERTISEMENT
Biotech innovator Biosyntia ApS, the latest addition to the ECBF, is applying proprietary biological processes to produce sustainable nutritional ingredients – a mission that has captured the imagination of seasoned biotech investors and major ingredient players.
Sweden’s Belach Bioteknik has been partnering mostly with researchers and pharma customers to design suited bioreactor systems for biomanufacturing. Most recently, the company has entered the growing cultured meat sector, in which many companies are currently scaling up their production and thus need expertise to get optimal results.
The Berlin-Brandenburg capital region is one of the leading life science regions in Europe and a lot is being done to keep it that way: with a wide variety of space offerings and support programs for founders and start-ups.
In soccer, such a transfer would raise tempers: A superstar leaves his team because he no longer trusts it to succeed. A warning sign, a headline event, fodder for weeks of debate in the media. Something similar has just happened to the biotech industry, but things remain strangely quiet in the fan block of Germany’s top research – whispers at best, just whispers, but no outcry anywhere.
The extension, which was celebrated with an official groundbreaking ceremony, will connect the IZB campus in Martinsried to the Munich subway system.
Toulouse White Biotech (TWB) is a good example to how scaling and collaboration through production partnerships can help the industrial biotech sector to satisfy the urgent need to speed up and scale sustainable processes for industrial production.
Researchers have presented a novel method to promote biofilm formation. They believe their method can help to increase the efficiency of biocatalysis in biotechnological production processes and thus allow cost-effective recycling of waste, as well as carbon dioxide conversion into chemical building blocks and natural gas,
or amino acids.
Last year, the G7 heads declared to end funding for the oil industry by the end of this year. At the COP27 in November, the WHO and United Nations renewed promises to redirect the hundreds of billions of dollars invested annually in fossil fuels to fund renewable energy initiatives.
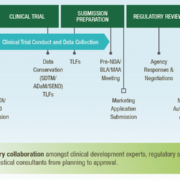
Strategic planning for the development of a novel therapy is critically important to reduce financial burdens, save time, and find the best, shortest path forwards. No two products will have the same pathway or same criterion for optimisation, but early, forward-looking planning can result in greater efficiency and a better understanding of key decisions and timepoints along the course of novel therapy development.