The renewable energy sector is ramping up in a major way, but a host of infrastructure issues still need to be solved. One is that supply at times now outstrips demand, but there’s no way to store excess power. Turning it into an easy-to-store, energy-rich source like methane could solve the problem. A pivotal aspect of power-to-gas’ technology harnesses some tiny helpers usually found in some of the most extreme regions on the planet.
ADVERTISEMENT
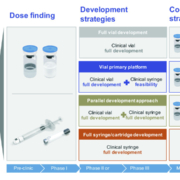
Considering the high development costs of medications and the time invested before a drug goes from discovery to market, it is critical to develop not only a solid drug development strategy, but a contemporary manufacturing strategy as well. A parallel development strategy could be one possible approach.
For years, neurologists have hunted for the source of cell toxicity that drives neurodegeneration and cognitive decline in Alzheimer’s disease. An Indian researcher at the University of Zurich might now have solved the mystery. It’s not hidden in amyloid plaques, as researchers previously believed, but in the microglia, the brain’s innate immune system, which triggers loss of synaptic junctions resulting in loss of cognitive functions.
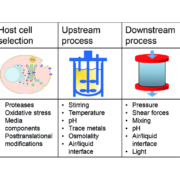
In the past, the role of customised and tailored formulations have been largely underestimated in the field of biopharmaceutical product development. An advanced formulation strategy can strongly improve the product, leading to significant customer benefits. The growing and increasingly competitive biopharmaceutical market requires individual solutions, particularly for better product stability and prolonged shelf life.
GARDP:?Six European states, together with the British Wellcome Trust and South Africa, have pledged €56.5m to help develop new antibiotics that break resistance. The ambitious goal of the Global Antibiotic Research and Development Partnership (GARDP) is to develop and deliver up to four new treatments that enter clinical development within the next six years.
Biomanufacturing?While the FDA and EMA have for the first time okayed the switch from batch to continuous manufacture of a pill – Janssen Cilag’s HIV protease inhibitor darunavir (Prezista) – the
industry is still waiting for such a signal in the biologics field. The FDA wants to boost adoption, and biopharma majors, such as Novartis, Amgen and Biogen, launched a pilot project to lay the groundwork for the next generation of production: continous USP and DSP, and alternatives to CHO cells.
Flexible and reliable contract manufacturing and development have become the key success factor in the development of biopharmaceuticals. Due to the long process time and high investment needed to build up production capacities, outsourcing of biologics manufacturing provides both access to advanced technologies and necessary capacity along with flexibility in timing and output.
Bioeconomy:?It could be a turning point for the fish meal and fish oil dependent animal feed industry in Europe. Since July, the European Commission has allowed the use of insect-processed animal proteins for aquaculture. According to experts, the use of insect meal as a sustainable alternative opens an US$90bn market.
Serialisation of drugs is confronting pharmaceutical companies all over the world with challenges, especially in relation to data handling and the integration of additional equipment and functions into existing packaging lines. Offline solutions, where secondary packaging is serialized directly by the folding-box manufacturer, combined with sophisticated data flow architectures, are a reliable, flexible, and immediately available alternative to inline serialisation.
Genetic Diseases:?It’s a disease that often strikes people in the prime of life, slowly destroying control over body, mind and personality. It inevitably kills the patient, but usually only after at least a decade of suffering. The underlying cause of Huntington’s disease – a dominant mutation on Chromosome IV – was discovered almost 25 years ago. Now the first trials to target the condition at its source have begun. Chances of finding a cure have never been more realistic.