An impulse paper of a green splinter group has ignited a storm in the water glass. Like industry and science lobbyists, the renegades are calling for changes to the EU’s legal GMO framework.
ADVERTISEMENT
A challenge whose success lies in efficient process management and digital development! The times of digitalisation and Industry 4.0 do not stop at the production of medical cannabis for the pharmaceutical industry. The combination of IT systems and blockchain-based processes allows the value-added chain to be automated and the data generated to be mapped in a tamper-proof manner.
The medical cannabis market has grown significantly in recent years. In Germany, the quantity of imported cannabis products has increased dramatically. In comparison to only 1.2 tons in 2017, German medical cannabis distributors imported about 3.13 metric tons of cannabis flowers for direct medical care of patients in 2018, while around 120 kg have been re-exported. In 2019, this amount doubled to around 6.7 tons.
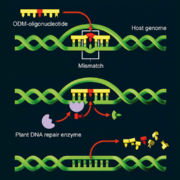
The European Commission has taken up the European Council’s demand for deregulation of new plant breeding techniques (NBT). A new study is being conducted to form the basis for a campaign from the Commission to inform the public about the benefits of targeted mutation technologies and to break the resistance to genetically engineered crops.
Manufacturers are keen to get involved in the alternative meat market. Investors and incubators are pushing forward start-ups.
A first wave of cultured meat companies is pushing forward. European start-ups such as Mosa Meat and Peace of Meat are part of the game.
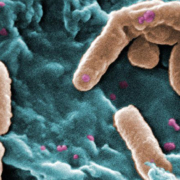
Austrian Phagomed wants to close a Series A financing in 2020. The start-up is working on phage-based treatments as a new alternative to antibiotics.
It’s a discovery that revolutionises our fundamental understanding of cells. Tiny droplets called condensates that form through weak interactions between proteins and RNA are at the heart of many key biological processes. Dewpoint Therapeutics – the first start-up to harness the basic research for a new therapeutic platform technology – is now opening a facility in the German capital Berlin.
With the coronavirus pandemic mounting, experts are warning of AMR as being the next, hidden crisis.
FGK Clinical Research GmbH opens a branch office in Berlin this month. The Berlin office will support the operative units at the German headquarters in Munich.