Shock: Adrenomed AG gets FDA fast track designation
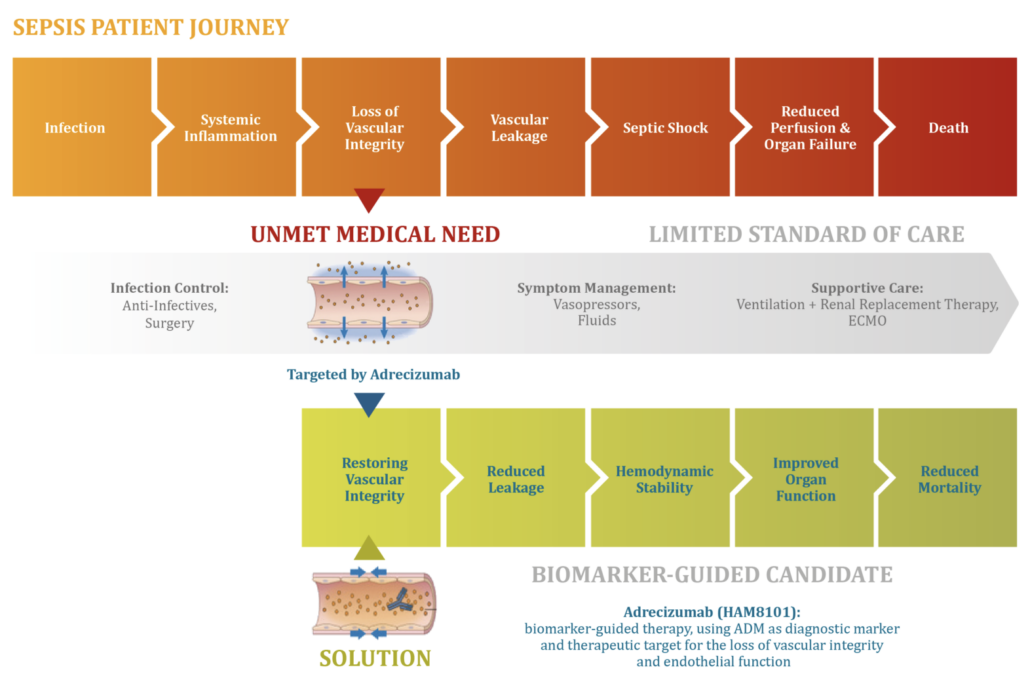
German AdrenoMed AG has got FDA Fast Track designation for its targeted septic shock medicine enibarcimab that restores endothel integrity.
After Adrenomed AG has demonstrated that its septic shock treatment led to a 60% reduction in relative 28-day mortality vs. placebo in a stratified patient group, the US FDA has granted fast track designation for its first-in-class antibody enibarcimab (previously adrecizumab). The company now heads for starting a pivotal Phase IIb study, achieving the original goal of Adrenomed founder Dr Andreas Bergmann to get approval after Phase IIb.
According to Adrenomed, enibarcimab acts like a hoover for the vasoactive 6kDA peptide adrenomedullin. Excess enibarcimab in the bloodstream binds most of the vasoactive 6kDA peptide adrenomedullin, which freely diffuses between the extra- and intravascular space, fixing it to the intravascular compartment and thereby restoring the barrier function of the endothelium. When located in the intravascular space, the peptide hormone causes vasodilation, which contributes to the rapid drop in blood pressure that precedes septic shock with multiple organ failure.
In preclinical studies, Adrenomed was able to show that its antibody greatly alleviates the symptoms of incipient septic shock by shifting the adrenomedullin balance to the intravascular space. In clinical follow-up studies including the AdrenOSS study (n= 301), the company showed that the response to the antibody was highest in patients with elevated levels of two blood biomarkers. High levels of a stable adrenomedullin fragment in the blood (ADM) indicate that the amount of intravascular adrenomedullin is increased. Elevated levels of the proprietary biomarker DPP3 of the diagnostics company Sphingotec indicate a reduced pumping capacity of the heart and impaired renal clearance associated with septic and cardiogenic shock. Last year, shock expert Prof. Peter Pickkers (Nijmegen) demonstrated that high levels of circulating DPP3 (cDPP3) indicate a high risk of organ dysfunction and mortality. This pathway is mechanistically independent from the loss of vascular integrity, which is known to be the main driver of mortality in septic shock and is indicated by elevated plasma levels of ADM (>70 pg/mL).
If approved, enibarcimab would have great market potential due to the lack of causative sepsis therapies. With a mortality rate of 20-30% for sepsis and 30-50% for septic shock in industrialised countries, sepsis is the cause of almost 20% of all deaths worldwide
Dr Richard Jones, CEO of AdrenoMed AG said after the FDA designation: "This is a great validation of AdrenoMed’s commitment to bring precision medicine to the intensive care unit to reduce patient heterogeneity and improve efficacy through targeted treatment."
In the AdrenOSS-2 study, patients with septic shock were treated with enibarcimab showed improvement in organ function and a significant reduction in 28-day mortality from 24% to 8% was achieved in adrenomedullin- and DPP3-positive patients. In the upcoming Phase IIb trial, the Hennigsdorf-based company wants to confirm this findings in a larger patient cohort.
"We are very confident that enibarcimab in combination with the two biomarkers adrenomedullin and circulating dipeptidyl peptidase 3 (cDPP3) has the potential to become the first effective and targeted treatment for septic shock. With AdrenoMed’s innovative biomarker-guided approach, it is possible to clearly define the patient population that will benefit most from enibarcimab, leading to a more pronounced treatment effect and improved mortality in septic shock," said Dr Stephan Witte, CMO of AdrenoMed.


 FDA
FDA
 Ipsen SA
Ipsen SA