Adrenomed reveals MOA of sepsis antibody
Adrenomed AG announced the publication of three scientific papers revealing how its adrenomedullin-targeting Phase II sepsis antibody Adrecizumab reverses vascular hyperpermeability in sepsis.
Data from preclinical and clinical tests reported in papers in Shock and Critical Care support the view that Adrecizumab acts through stabilisation of the vasoactive peptide hormone adrenomedullin and its redistribution from tissue into blood plasma while adrenomedullin receptor signaling remains intact. The resulting stabilization of the endothelial barrier function and vessel tone counteracts vascular leakage, which contributes to the blood pressure drop preceding septic shock. In fact, clinical and preclinical data show that Adrecizumab-induced rise in adrenomedullin plasma levels attenuates vascular leakage, improves kidney function and reduces mortality. According to Adrenomed, the completely new mode of action of Adrecizumab (HAM8101) could lead to significant improvements in the treatment of sepsis.
The team headed by Prof. Peter Pickkers (Department of Intensive Care Medicine, and Radboud Center for Infectious Diseases) from Radboud University Medical Center, Nijmegen, The Netherlands) showed in two animal models for sepsis and in humans with systemic inflammation show that Adrecizumab restores the impaired vascular barrier function that causes hemodynamic instability in sepsis and leads to edema formation, organ failure and shock:
- Excess Adrecizumab in the blood vessels binds most of the vasoactive 6kDA peptide adrenomedullin, which freely diffuses between the extra- and intravascular space, fixing it to the intravascular compartment where adrenomedullin restores the barrier function of the endothelium.
- Excess of Adrecizumab does not increase adrenomedullin synthesis in smooth vascular muscle and endothelial cells.
- Adrecizumab (plasma half-life 14d), which binds adrenomedullin N-terminally, prolongs the short half-life (22 mins) of the free peptide in the vasculature by protecting it from N-terminal proteolysis while only marginally affecting its C-terminal receptor binding activity in the endothelium.
- Redistribution of adrenomedullin from the extravascular space to the intravascular compartment promotes adrenomedullin’s proven intravascular effects (closing of endothelial gaps, tight junctions) while preventing its extravascular effects (vasodilation).
The proposed mode of action is supported by new animal data, published in Shock [2]. In a rat endotoxemia model (N=48) administration of Adrecizumab before induction of sepsis by bacterial lipopolysaccharide (LPS) attenuated LPS-induced vascular hyperpermeability by 40% to 71% vs controls as measured by albumin leakage into the kidney. In a cecal ligation and puncture (CLP) mouse model for sepsis (N=24), a single Adrecizumab injection prior to surgical induction of sepsis also significantly reduced albumin leakage into renal tissue (77-78% vs placebo). In an additional study with CLP mice (N=60), a single injection of Adrecizumab reduced 7-day mortality by 50%, while repeated injection of the humanized antibody reduced 14-day mortality by 60%. The results provide statistically significant evidence that Adrecizumab prevents inflammation-induced vascular hyperpermeability and improves survival in mouse models for sepsis.
Endothelial dysfunction and vasodilation are hallmarks of sepsis, commented Dr. Andreas Bergmann, Founder and Chief Scientific Officer of Adrenomed AG. The unique mode of action of Adrecizumab has large potential to add significant benefit to the treatment of sepsis and early septic shock. An interim analysis of a proof-of-concept Phase II study with Adrecizumab in 300 patients with septic shock is expected in Q4/2018. Furthermore, Adrenomed is planning a Phase II proof-of-concept study in patients with acute decompensated heart failure.
Currently, there is no treatment available able to improve outcomes in septic shock. While Adrecizmab has a way to go to reach the market, German diagnostics maker sphingotec GmbH announced at the end of May that it acquired a POC platform for a test that diagnoses patients at high risk to develop septic shock via elevated blood levels of adrenomedullin (bio ADM). We are delighted to shortly start European distribution of our acute care biomarkers for therapy monitoring and adjustment on a great POC testing platform, said Dr. Andreas Bergmann, founder and CEO of sphingotec GmbH. sphingotec GmbH will offer its next-generation sepsis, heart failure and acute kidney injury biomarkers penKid® and bio-ADM® on the FDA-approved NEXUS IB 10 point-of-care (POC) testing platform, originally developed by Nexus Dx. POC testing of the sepsis marker bio ADM (and the kidney function marker penKid) is set to add significant clinical benefit to emergency departments and intensive care units allowing on-site diagnosis, monitoring, and guidance in therapies of sepsis, decongestion in heart failure and acute kidney injury.


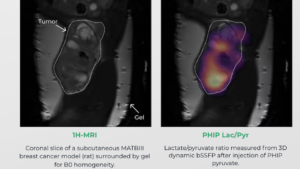 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics