Novimmune enters US$195m option deal with TG Therapeutics Inc.
Swiss Novimmune SA has exclusively sold a licence option on its lead product NI-1701 (now renamed TG-1801), a bispecific anti-CD47/anti-CD19 antibody designed to trea B-cell blood cancers, to TG Therapeutics Inc.
Under the exclusive option agreement, TG Therapeutics pays an undisclosed up-front fee and holds out US$185 in future milestones plus royalties on net sales. However, both TG Therapeutics and Novimmune will each maintain an exclusive option, exercisable at specific times during development, for TG Therapeutics to license the rights to NI-1701. TG Therapeutics and Novimmune will be jointly responsible for all development and commercialisation costs of the product.
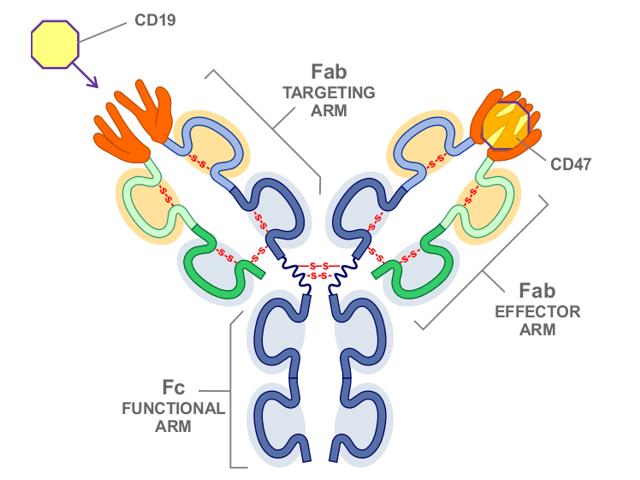
NI-1701, a human IgG1-like bispeciufic antibody, dis based on Novimmune’s -body format. One mechanism unique to this bispecific antibody involves blocking of CD47 referred to as the do not eat me signal for the body’s phagocytic cells specifically directed to CD19 positive cells. The net effect is highly targeted, potent anti-B-cell tumor phagocytic activity, while avoiding the general toxicity concerns associated with earlier agents targeting the CD47 pathway. Moreover, the co-targeting of CD19 is not only expected to enhance safety but by retaining its IgG1 Fc functionality, this agent is designed to provide a secondary mechanism of anti-tumor activity through the induction of antibody dependent cellular cytotoxicity (ADCC).
Novimmune expects TG-1801/NI-1701 to be the first anti-CD47 bispecific antibody to go into clinical trials this year or early in 2019.Various solid and hematologic cancers exploit CD47 expression in order to evade immunological eradication, and its overexpression is clinically correlated with poor prognoses. We are delighted to see our first bispecific antibody move forward into the clinic with an experienced partner in the field of hematological malignancies, and to provide proof of principle for our completely novel approach, said Chairman and Chief Executive Eduard Holdener.
"TG-1801 has demonstrated encouraging pre-clinical anti-tumor activity both as a single agent and in combination with anti-CD20 monoclonal antibodies," stated Michael S. Weiss, Executive Chairman and CEO of TG Therapeutics. "With the addition of TG-1801 to our pipeline, we now have three targeted immunotherapies in-house that can potentially be used together to create a novel non-chemo treatment option that uses the body’s immune system to fight B-cell cancers, including NHL and CLL.
TG Therapeutics is developing two therapies targeting hematological malignancies and autoimmune diseases. Ublituximab (TG-1101) is a glycoengineered monoclonal antibody that targetting a unique CD20 epitope on mature B-lymphocytes. TG Therapeutics is also developing umbralisib (TGR-1202), an orally available PI3K delta inhibitor. Both ublituximab and umbralisib, or the combination of which, are in Phase 3 clinical development for patients with hematologic malignancies, with ublituximab also in Phase 3 clinical development for Multiple Sclerosis.
Novimmune has licenced several pipeline products to pharma companies including Genentech and Shire, now Takeda.



 Unsplash+
Unsplash+