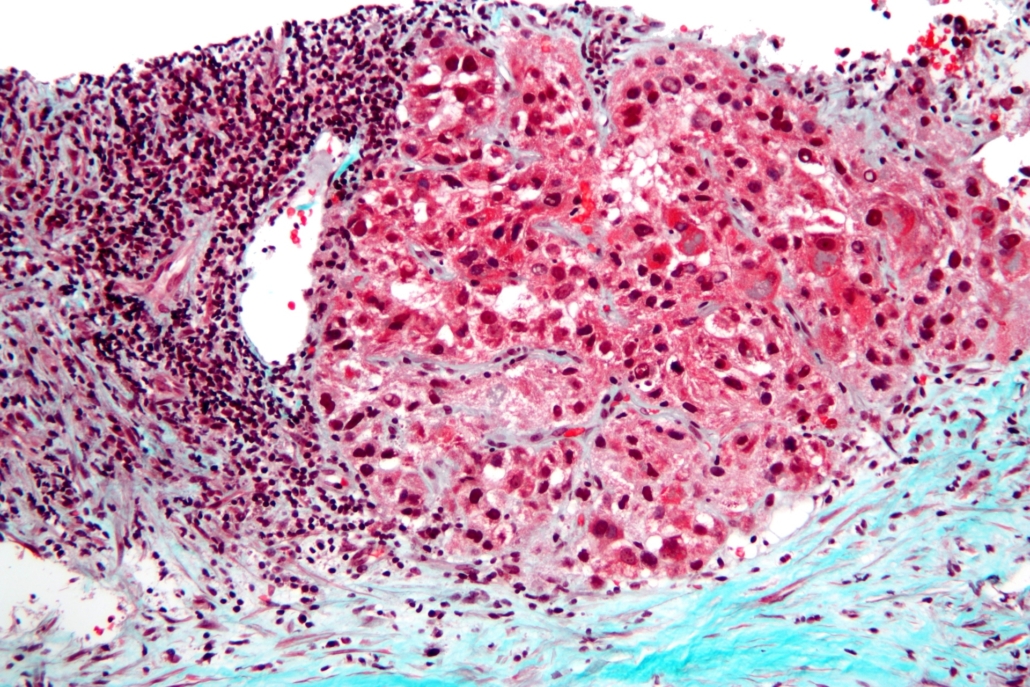
Tecentriq-Avastin combo hits endpoint in liver cancer
Roche AG said on Friday that its PD-L1 blocker Tecentriq plus its VEFG blocker Avastin improved progression-free survival versus sorafinib in a pivotal study in hepatocellular carcinoma (HCC) patients.
Data from 501 treatment-native HCC patients enrolled in the open label IMbrave150 Phase III study demonstrated a incremental improvement of progression-free survival (PFS) in the verum arm (atezolizumab + bevacizumab. 6.8 months) versus sorafenib (4.3 months). Median overall survival, however, was not yet reached after median follow-up 8.6 months vs sorafinib (OS: 13.2 months). With 750,000 patients diagnosed annually, HCC is most common form of liver cancer.
Based on statistical estimates, however, Roche claimed IMbrave150 to represent the first Phase III cancer immunotherapy study to show an improvement in overall survival. Tecentriq in combination with Avastin reduced the risk of death (OS) by 42% (hazard ratio [HR]=0.58; 95% CI: 0.42-0.79; p=0.0006) and PFS by 41% (HR=0.59; 95% CI: 0.47-0.76; p<0.0001), compared with sorafenib, said Roche in a press release.
For the first time in a decade, we are seeing a treatment that has improved overall survival for people with unresectable hepatocellular carcinoma compared with the current standard of care, said Levi Garraway, new Chief Medical Officer at Roche and Head of Global Product Development. He suggested Tecentriq in combination with Avastin could transform the treatment of this aggressive disease, and we are working closely with global health authorities in the hope of bringing this treatment option to patients as soon as possible.
As Roche’s Phase III study has an estimated completion data at the end of June 2022, it might be the first checkpoint blocker plus targeted therapy combo seeking regulatory approval. Immuno-oncology market leader Merck & Co Inc, however, will complete its Phase III Leap-002 trial with anti-PD1 Keytruda plus kinase blocker Lenvima in July 2022. Bristol-Myers Squibb Co. will follow with a combination therapy of anti-PD1 plus anti-CTLA4 Opdivo in September 2023.


 International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s
International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s
 Roche
Roche