
Ichnos Sciences in licence deal with Almirall
Ichnos Sciences Inc. has licenced the gobal rights on its IL-1RAP antagonistic antibody ISB 880 to Almirall SA for the treatment of autoimmune disorders.
Under the agreement, Almirall will assume the global rights to develop and commercialise the monoclonal antibody for the treatment of autoimmune diseases. Glenmark’s US-based subsidiary Ichnos will retain the rights for antibodies acting on the IL-1RAP pathway in all oncology indications.
Almirall will assume full cost and responsibility for the global development and commercialisation of the antibody in several autoimmune disease indications. Ichnos will receive an upfront payment of €20.8m as well as additional development and commercial milestone payments and tiered royalties based upon future global sales.Financial details were not disclosed.
ISB 880 is a first-in-class fully human monoclonal antibody that targets human IL-1RAP. Blockade of IL-1RAP simultaneously abrogates multiple disease drivers among the IL-1 family of proinflammatory cytokine receptors, including IL-1R, IL-33R, and IL-36R, differentiating ISB 880 from single cytokine blockers. These cytokines have been implicated in numerous autoimmune conditions, opening opportunities for ISB 880 to be positioned across broad disease indications. IND-enabling work of the preclinical compound has been conducted and filings with regulatory authorities are under development.


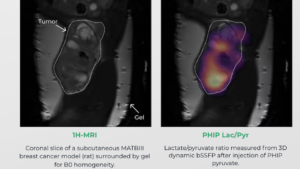 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics