New tools to support AAV manufacturing
With the rapid developments in the field of gene therapy, the use of viral vectors, especially AAV, to treat various monogenic, inherited diseases in human patients is gaining momentum. Nonetheless, there are still options to optimise the production of such vectors in mammalian cells.
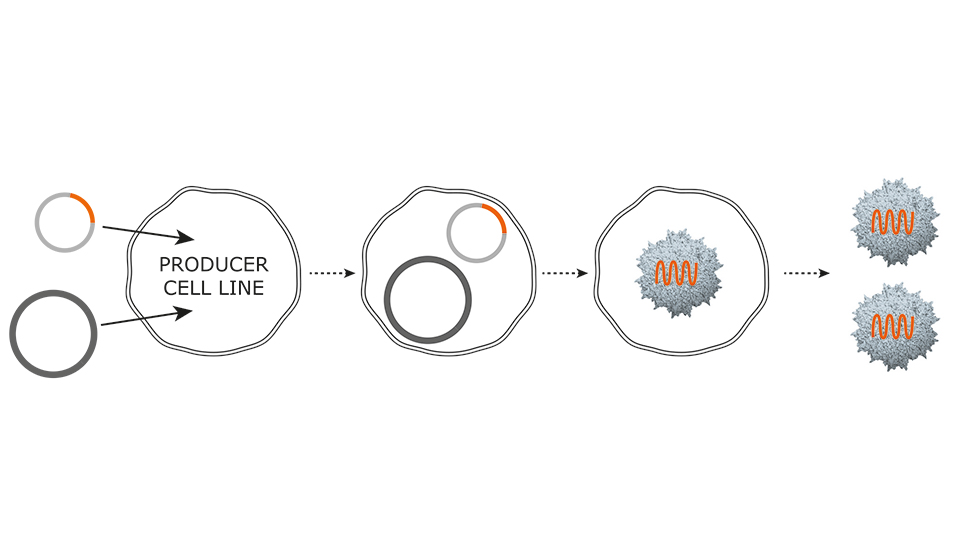
For the production of AAV vectors, PlasmidFactory offers worldwide exclusively a 2-plasmid-system for transfection of producer cell lines (e.g. HEK293), replacing the old-fashioned 3-plasmid-system and causing a significant cost-of-good-advantage for the user.
2-Plasmid-System
One construct carries the AAV packaging functions such as rep and cap and the adenoviral helper functions. On the second construct, the eukaryotic gene of interest is present, flanked by Inverted Terminal Repeat (ITR) sequences.
Sequence integrity of these ITRs is crucial for successful amplification of high viral titers. However, ITRs are very unstable during plasmid replication. Depending on the culture conditions applied, this results in a fraction of plasmids, containing shortened, non-functional ITRs which are useless in AAV production.
ITR rescue
The standard quality control for ITR sequences is a restriction digest with SmaI. However, this is still not 100% accurate in detecting sequence changes for two reasons: a) very small restriction fragments are hard to detect in agarose gel electrophoresis. b) the regions of the ITR not covered by the restriction sites may go undetected after a mutation. Furthermore, the fraction of not-intact plasmids cannot be quantified accurately.
Conventional Sanger sequencing is used to check the overall sequence integrity of finished plasmids before release. However, the ITRs are notoriously difficult to sequence, which may yield indistinct results.
By a special type of next-generation sequencing, established by PlasmidFactory’s team, the ITR sequences in AAV transfer plasmids can be sequenced and quantified, complementing PlasmidFactory´s established fermentation techniques to maintain intact plasmids.
AAV minicircles (MCs)
Improvements on the DNA level also lead to a significantly higher yield in AAV manufacturing: The development of minicircles to replace plasmids for production of AAV vectors. As bacterial backbone sequences only needed for amplification in E.coli are removed during MC production, encapsidation of prokaryotic plasmid backbone sequences is avoided. This is of particular importance for scAAV vector preparations, which contain an unproportionatey high amount of plasmid backbone sequences. Replacing plasmids by MC constructs further improves efficiencies of AAV preparations. Thus, MC technology offers an easy to implement modification of standard AAV packaging protocols that significantly improves AAV vector preparations.
This article was originally published in European Biotechnology Magazine Winter Edition 2021.



 Unsplash+
Unsplash+