After a turbulent phase: a new milestone from Takeda for Heidelberg Pharma
Positive signals from a partnership with Takeda are allowing Heidelberg Pharma to push the setback from a milestone payment not received as planned from Telix somewhat into the background. Nevertheless, the company has been strongly shaken and is now hoping for calmer waters in order to drive its clinical development forward with greater focus.
Job cuts, restructuring, a narrowed focus on the drug pipeline … and a new CEO, after the previous one was blamed for the significant delay of a planned milestone from a diagnostics partnership. This was anything but a peaceful pre-Christmas period in Ladenburg near Heidelberg. After months of turbulence, a glimmer of hope is now emerging at Heidelberg Pharma. Only shortly before Christmas, the newly appointed CEO Dr Dongzhou Jeffery Liu had prepared shareholders for painful cutbacks: the delayed milestone payment and, with it, the central question of financing dominated the picture.
Milestone from partner Takeda
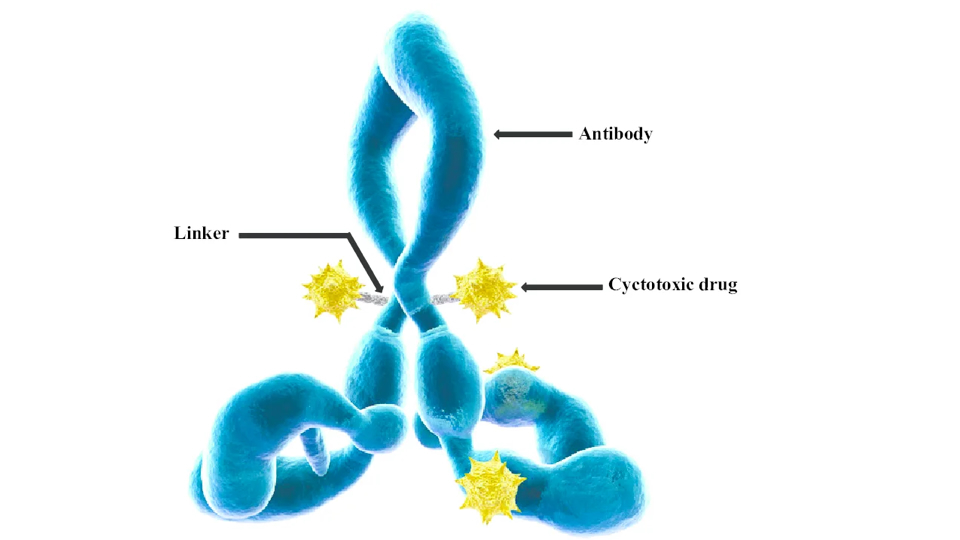
There is now a positive signal from the partnership with Takeda. Under the existing licence agreement, the Japanese pharmaceutical group has made a milestone payment to Heidelberg Pharma, triggered by the dosing of the first patient in a Phase I/II clinical trial with an ADC candidate based on the ATAC technology. While no financial details were disclosed, the payment underlines operational progress beyond the company’s own pipeline. However, this milestone should not be confused with the one expected – and still expected – from the Australian company Telix, which, in the event of FDA approval of the renal cancer diagnostic originally developed by Munich-based Wilex AG, is likely to be of a significantly higher magnitude than a payment from the early development stage with Takeda. Heidelberg Pharma first had to gain, and in effect insist on, better access to information on the delays and the status of TLX250-CDx at Telix. Communication between the partners has since improved.
The light on the horizon has thus become more visible again for Heidelberg Pharma and may help dispel the low-hanging clouds of a spoiled Christmas mood. There are now three ATAC-based candidates in clinical development – two proprietary programmes at Heidelberg Pharma and one with a strong partner. Dosing patients with the ADC and a toxin derived from the death cap mushroom has proven highly promising, as relatively high concentrations could be reached without severe side effects, and alongside good responses, complete remissions have already been observed in two out of four patients.
Back to work and waiting for next data from ongoing clinical trial
Dr Dongzhou Jeffery Liu, Chief Executive Officer of Heidelberg Pharma AG, who moved from partner Huadong Medicine to Heidelberg Pharma commented: “We are very pleased that our partner Takeda is advancing the development of its candidate based on the ATAC technology and that we have received the corresponding milestone payment.” For the company, the feedback from Takeda is more than just a financial inflow: it is an important external vote of confidence in a technology that appears to be regaining its footing after difficult months.



 BioNTech SE
BioNTech SE Roche
Roche