Calluna Pharma starts efficacy test with unique IPF antibody
Norway-based Calluna Pharma A/S has initiated patient recruitment in late August for its Phase II AURORA trial investigating safety and efficacy of CAL101, a monoclonal antibody with unique mode of action to cure idiopathic pulmonary fibrosis.
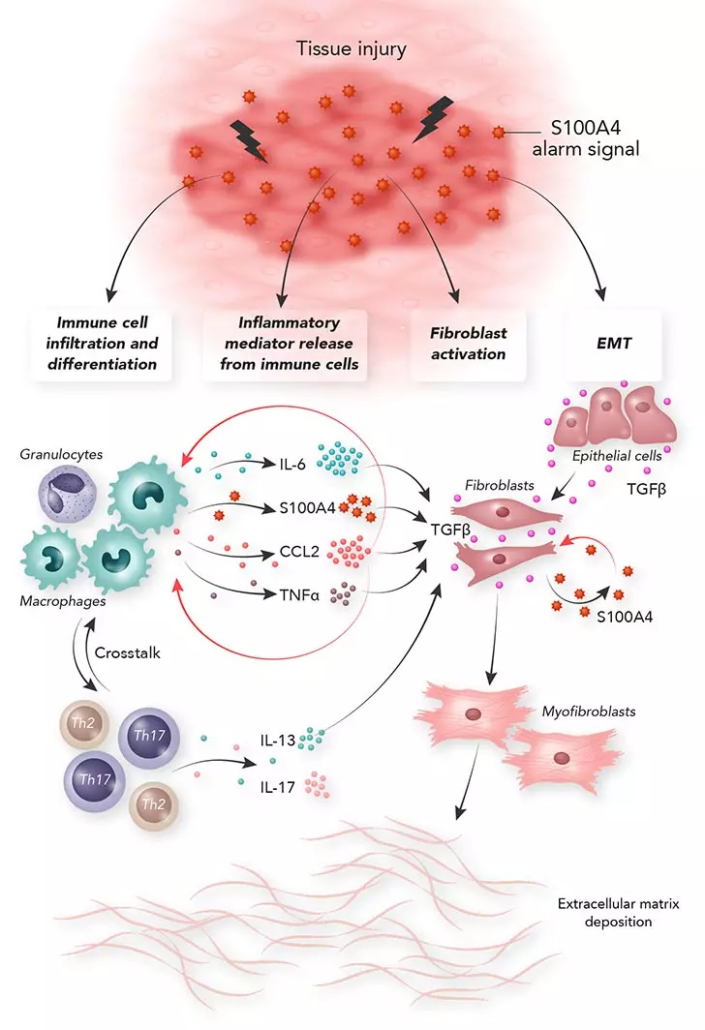
Among the 110 drug development programmes currently underway worldwide targeting the US$4.5bn IPF market, Calluna Pharma‘s IgG1 antibody directed against the damage-associated molecular pattern (DAMP) protein S100A4 stands out for its unique mechanism of action. Preclinical studies demonstrated selective inhibition of both fibroblast activation and pro-fibrotic inflammation, alongside a favourable safety and immunogenicity profile, which was confirmed in Phase I trials. In preclinical models, CAL101 halted IPF progression and restored tissue homeostasis by deactivating signalling pathways involved in persistent and maladaptive scar formation. IPF has a median survival time of three to five years.
The multicentre Phase II safety and efficacy study conducted in partnership with Lilly ExploR&D is set to enrol 150 individuals with IPF. Following a 28-day screening period, patients will be randomised in a 3:2 ratio to receive seven monthly intravenous infusions of either CAL101 or placebo. The primary endpoint is lung function, measured by forced vital capacity (FVC), that is the volume of air that can be forcibly exhaled, compared to baseline.
Competitor products at a more advanced stage of clinical development are targeting either TGF-beta signalling pathways, LPA1 receptors, or CTGF. While these strategies are biologically relevant and based on sound antifibrotic rationale, their development faces several systematic challenges at molecular, pharmacological, and clinical levels. Drug candidates that block TGF-beta activation – including Pliant Therapeutics’ programme Bexotegrastintegrin (TGF-beta activation, Phase IIb/III) and Toray Industries’ inhaled TGF-beta1 siRNA TRK-250 (Phase I completed)– do address a master regulator of fibrosis. However, due to its pleiotropic activity, previously studied candidates such as galunisertib and fresolimumab were associated with gastrointestinal, cardiovascular, and haematological adverse events. Furthermore, TGF-beta must first be activated by integrins, and the presence of three isoforms of TGF-beta complicates selective targeting.
Preclinically, LPA1 blockers such as Bristol Myers Squibb’s BMS 986278, currently under evaluation in Phase III trials, led to reductions in both fibrosis and inflammation, similar to CAL101. However, the showed receptor redundancy, systemic adverse effects and, early toxicological setbacks.
Pamrevlumab, an antibody inhibitor of connective tissue growth factor (CTGF) developed by FibroGen and tested in Phase III trials, targets pathways downstream of TGF-beta. As a result, its effects may be circumvented by other pro-fibrotic signals and could potentially require combination with additional therapeutic mechanisms. Unlike the other two agents, CTGF inhibition may reduce symptoms but does not necessarily address the underlying inflammatory microarchitecture.


 EC- press service
EC- press service AseBio
AseBio adobe stock photo - Toko
adobe stock photo - Toko