Adaptimmune nears second approval for TCR-T therapy
After meeting the primary endpoints of a pivotal Phase II trial, UK-based Adaptimmune Ltd. is submitting an FDA Biologics License Application for its autologous T-cell receptor therapy (TCR-T) Lete-cel (letetresgene autoleucel).
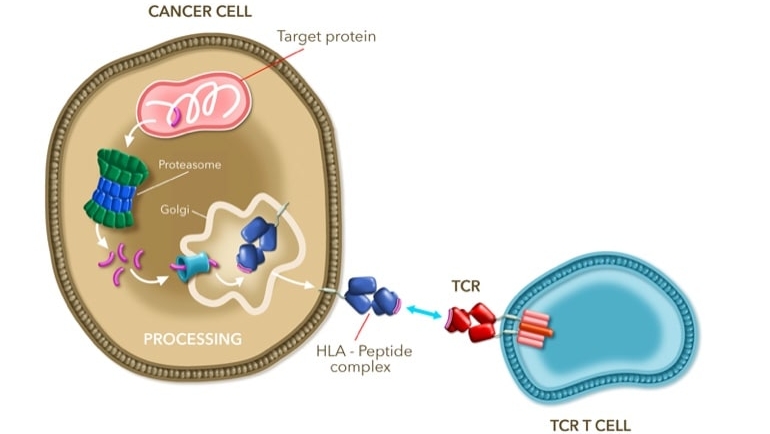
In contrast to CAR-T cell therapies, TCR-T cell therapies can be directed not only against membrane-bound tumour associated antigens but peptide fragments from intracellular natural cancer antigens. For this purpose, natural T cell receptors are affinity-optimised and their genes are transferred in vitro into the patient’s own CD8 or CD4 T cells previously isolated by leukapheresis, which are then multiplied.
In a modified pivotal study IGNYTE-ESO, Oxford-based Adaptimmune’s TCR-T cell therapy Lete-cel directed against the tumour antigen NY-ESO-1 achieved a response rate in 42% of the 64 evaluable patients with NY-ESO-1-positive advanced or metastatic synovial sarcoma or with myxoid/round cell liposarcoma (MRCLS). Six complete and 21 partial remissions were achieved. The UK company plans to submit its second marketing authorisation application (rolling BLA) after afami-cel for a second-line TCR-T therapy in solid tumours to the US Food and Drug Administration (FDA) by the end of 2025.
The primary data analysis showed a response rate of 14/34 (41%) in patients with synovial sarcoma and 13/30 (43%) in patients with MRCLS. The median duration of response was 12.2 months (95% CI 6.8, 19.5). In synovial sarcoma, the median duration of response was 18.3 months (95% CI 3.3, -). In MRCLS, the median duration of response was 12.2 months (95% CI 5.3, -). The median progression-free survival (PFS) was 5.3 months (95% CI 4.0, 8.0). Side effects such as cytopenias and cytokine release syndrome, which can prevent paediatric use, occurred most frequently, but are common and manageable with this class and generation of drugs.
In August this year, Adaptimmune received the world’s first accelerated FDA approval for its genetically engineered TCR-T therapy afami-cel (afamitresgene autoleucel) against solid tumours – more precisely: inoperable synovial sarcomas – on the basis of response rate, which must be confirmed by further clinical data. afami-cel is also directed against the cancer testis antigen Mage4
Following Roche’s withdrawal from a US$3m licence agreement, Adaptimmune was only able to obtain marketing authorisation for afami-cel and pay the costs of the IGNYTE-ESO trial because Galapagos NV stepped in as a partner. The Belgians licensed a third British IND-stage TCR-T candidate, uza-cel (Uzatresgene autoleucel), which recognises the MAGE-4A antigen on tumour cells, for an upfront payment of US$100m.


 Araris Biotech AG
Araris Biotech AG
 Roche
Roche