Modus Therapeutics AB, a company developing innovative treatments for patients with high unmet medical needs and a focus on sickle cell disease (SCD), announces the appointment of Mats Blom as Chief Financial Officer (CFO).
ADVERTISEMENT
Researchers at Janssen Pharmaceutical Companies of Johnson & Johnson in Leiden have developed a small molecule antibody mimic, that prevents entry of multiple influenza virus serotypes.
Seventure Partners has announced the first close of its second microbiome fund designed to put €200m in 20 companies developing microbiome-based health solutions.
Miranda Wolpert, MBE, an expert on child and adolescent mental health has joined the London-based Wellcome Trust as head of their mental health priority area.
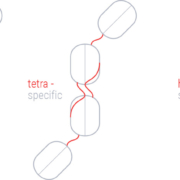
Swiss immunology and immuno-oncology specialist Numab Innovation AG has licenced its tri-specific antibody ND016 to Boston-based Intarcia Therapeutics Inc.
Biogen has strengthened its portfolio with Nightstar Therapeutics, a British gene therapy specialist for rare inherited eye disorders.
The EMA‘s human medicines committee (CHMP) has recommended eight medicines for approval in its last meeting in London, two thereof orphan medicines. Three got conditional marketing authorisation.
According to the FMD 2011/62, serialisation of all prescription drug packs in Europe will inevitably become a daily routine for the pharmaceutical industry from 2019. European Biotechnology spoke with Philip Falkenstein, Product Manager at Bähren Druck, about the use of pre-serialised labels as solution for special implementation areas and the current legal framework.
High-potency drugs present numerous opportunities for improved drug selectivity, efficacy and safety profiles, and represent a growing area of global development focus, for applications such as oncology and hormone-based treatments. Although this class of molecules offers numerous benefits to patients, they require particular care and attention in ensuring the safety for those involved in their manufacture and handling.
Neurimmune today announced the appointment of John Dellapa as General Counsel. John joins Neurimmune from the Boston office of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, an AmLaw 100 law firm.