Orphan drug leader Shire plc has been granted EU marketing authorization for its recombinant van Willebrand factor (rVWF) vonicog alfa as second-line treatment for van Willebrand disease, the most common blot clotting disorder..
ADVERTISEMENT
F-star, a biopharmaceutical company developing novel bispecific antibodies, today announces the appointment of Nessan Bermingham, Ph.D. as Chairman of its Board of Directors. Nessan succeeds John Edwards who has been F-star’s Chairman for seven years and will remain as a non-executive director.
Crescendo Biologics Ltd (Crescendo), today announced that Pavel Pisa, MD, PhD, has been appointed as Chief Medical Officer of the Company with immediate effect.
Drug Developers dealing with PD1-PDL1 checkpoint inhibitors must consider new safety problems associated with the hyped cancer meds.
The European Investment Bank (EIB) has issued its first Sustainability Awareness Bond worth €500m to support investments into water projects – health and nutrition to follow.
One of the world’s largest and most controversial mergers in the past few years was sealed in early June. German pharma and chemistry company Bayer has taken over US competitor Monsanto, forming the largest integrated provider of seeds, agrochemicals and digital farming solutions on the planet. The acquisition is part of a recent US$170bn deal binge that is already having a profound impact on the future of global agriculture.
Dunn Labortechnik will present a variety of cell culture products and the new Flexcell® FX-6000 Tension System from the American company Flexcell International at the International Meeting of the German Society for Cell Biology (DGZ) from 17th to 19th September, on booth no. 5 in the auditorium building of the University of Leipzig.
Around 300 international AMR experts met at ESCMID-ASM drug development conference in Lisbon to discuss the latest progress in the field.
Oxford-based Adaptimmune Therapeutics plc has raised US$100m in a registered direct offering of American Depositary Shares (ADS).
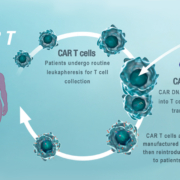
The NHS England announces it will reimburse Novartis’ CAR-T cell therapy Kymriah as treatment for children with B cell acute lymphoblastic leukemia (ALL) that are refractory, in relapse post-transplant or in second or later relapse.