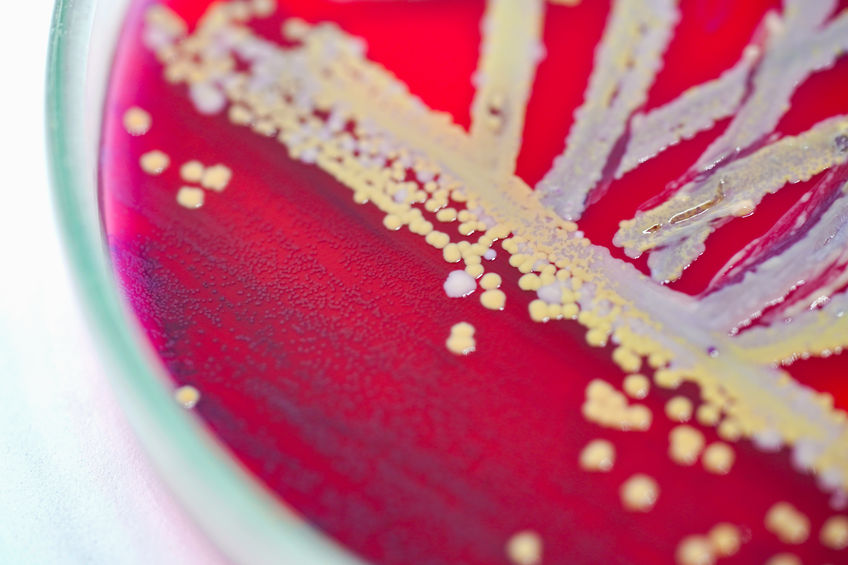
UK launches new payment model for antimicrobials
In order to incentivise drug companies to re-enter development of antibiotics, the UK government announced it will establish a licence model to assure developers sufficient return on investments (ROI).
A 5-year plan to tackle antimicrobial resistance (AMR) published by the UK government has ambitious goals. Using electronic prescription, it aims at reducing the use of antibiotics in humans by 15%. Additionally, the government wants to gain full control over AMR by 2040.
As pharma companies have quit the market for antibiotics due to lack of ROI, governments across the world think of how bring them back into development to fight AMR, which is due to overuse of antibiotics and genetic adaption of microbes to them. On the one hand, funds have been set up to incentivise screening of drugs with new mechanism against the most resistant bugs. The other part of the initiatives aimed at re-incentivising antibiotics development is new payment models.
The NICE, and NHS England have now announced a value-based licence model in which drug developers would be paid based on how valuable the medicines are to the NHS, rather than by the quantity of drugs sold. The NICE would lead the health technology assessment of antimicrobials. Work to introduce the new payment model will be underway within six months, said Britains Health State Secretary Matt Hancock at the World Economy Forum 2019 in Davos, Switzerland, adding the expectation that this will encourage companies to invest in the estimated £1bn needed to develop a new drug.
The plan is to licence a predefined amount of antimicrobials annually instead of purchasing them. This would allow to decouple ROI from the amount of antimicrobials prescribed. According to Hancock, The NHS would pay the price upfront. Additionally, the establishment of a national investment charge for antibiotics is discussed, which developers could either pay or invest. Last June, FDA Commissioner Scott Gottlieb proposed a similar model under which hospitals would licence antimicrobials.
The AMR community – and many of the small and mid-sized companies active in the field – welcomed the move to finally test a Pull mechanism. "It is very good to see action after many years of reports and we thank the UK government for making the first move", said Marc Gitzinger, CEO of Swiss Bioversys and Vice-President of the BEAM Alliance, representing AMR-focused SMEs in Europe. Marc Lemonnier, CEO of French Antabio and also BEAM Alliance Board member, added: "This announcement is a clear, unprecedented commitment to implementing a de-linked antibiotic purchase model. The UK leadership in raising the profile of this major threat to humanity is to be applauded." Also from the US, the UK initiative received support. "A monumental advance for the field", said Kevin Outterson, executive director of US-based CARB-X. And John Rex, one of the major international AMR key opinion leaders, commented in his newsletter: "That we have arrived at this exciting step is due to the work of the many, many hands behind DRIVE-AB, the O’Neill-led UK AMR Review, the Duke-Margolis-led reviews, and more. There’s of course more work to be done and many questions to resolved: Which antibiotics to buy? How much to pay? How to pay? What duration of purchase? But at least we are at the stepping off point for a new approach to reimbursing antibiotics."
New payment models for antimicrobials to address the broken business model in the AMR field will be a key topic at the 12th Berlin Conference on Life Sciences "Novel Antimicrobials and AMR Diagnostics 2019". Representatives from NICE and Wellcome Trust will provide more insights about the new UK initiative in the "Market Access" session on Day 2 chaired by the Global AMR R&D hub.
Organiser BIOCOM expects 350 international experts in AMR to attend to the 2-day conference which is supported by UK Trade and Invest, the European ENABLE consortium, GARDP, CARB-X, Novo REPAIR Impact Fund and Biomérieux.


 DiogenX SA
DiogenX SA Adaptimmune
Adaptimmune Qiagen NV
Qiagen NV