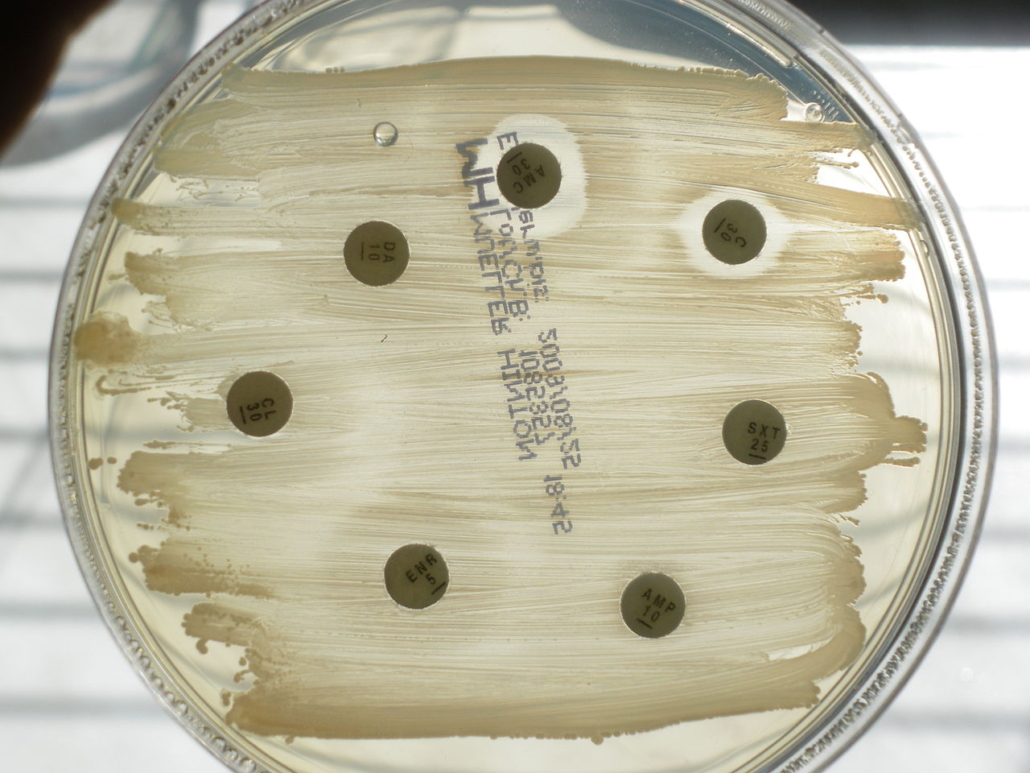
AMR: FDA provides financing model for novel antibiotics
As first regulatory body in the world, the FDA has presented a market-driven reimbursement mechanism for antibiotics that may incentivise drug development in a niche market with currently low return on investment.
The new reimbursement model could be a blueprint for other healthcare systems whose stakeholders have not yet made a decision on a financing model for antimicrobials than can break therapy resistance or last resort antibiotics, which are prescribed sparsely.
The US FDA, CMS and other agencies have started talks how to change the reimbursement model for antibiotics to create an incentive for the development of new classes of antimicrobials. For resistance-breaking new drugs, they consider a model such as for software. According to that model, payors would have to pay a licensing fee for a predefined annual amount of doses instead of reimbursing on a per-use basis. This would ensure a predictable return for drug developers, FDA Commissioner Scott Gottlieb said yesterday. However, experts have doubts that the cash-strapped hospitals will accept such upfront payments even if reimbursable.
Traditional reimbursement models which link the ROI to sales volumes didn’t work with antimicrobials because resulting overprescription would increases the probability of mutations that lead to drug resistance. The FDA is also working to make clinical testing of new antimicrobials easier and cheaper. This would improve the ROI for companies thus making the market more interesting to drug developers. European antibiotics experts will meet on 14-15 March 2019 at the Berlin Conference to find new reimbursement models for antimicrobials and to discuss the best push and pull mechanisms to re-vive the market for antimicrobials.



 SANOFI
SANOFI