Kinase inhibitors: Promising therapeutics for neurodegenerative diseases
Neurodegenerative diseases like Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS) suffer from a scarcity of effective treatments. Current therapies primarily provide symptomatic relief but do not address the underlying pathology, failing to slow or halt disease progression. This underscores the urgent need to identify new therapeutic targets relevant to the pathology of these conditions. Kinases represent highly tractable drug targets, with kinase inhibitors achieving significant success, particularly in oncology. However, their potential in neurodegenerative diseases remains largely unexplored, presenting a promising avenue for future research and therapeutic development.
The rationale for targeting kinases in central nervous system (CNS) drug discovery is based on the understanding that phosphorylation, catalyzed by kinases, plays a pivotal role in regulating many cellular processes, and that at least a number of phosphorylation processes may become aberrant in disease (Figure 1). Over the past decade, there has been an increasing interest in the therapeutic potential of brain penetrant kinase inhibitors for neurodegenerative diseases. The development of kinase inhibitors, such as glycogen synthase kinase-3β (GSK-3β) inhibitors for AD, leucine-rich repeat kinase 2 (LRRK2) inhibitors for PD, and mitogen-activated protein kinase kinase kinase kinase (MAP4K) inhibitors for ALS, has shown promise, though challenges like specificity, blood-brain barrier penetration, and drug resistance remain 1-5.
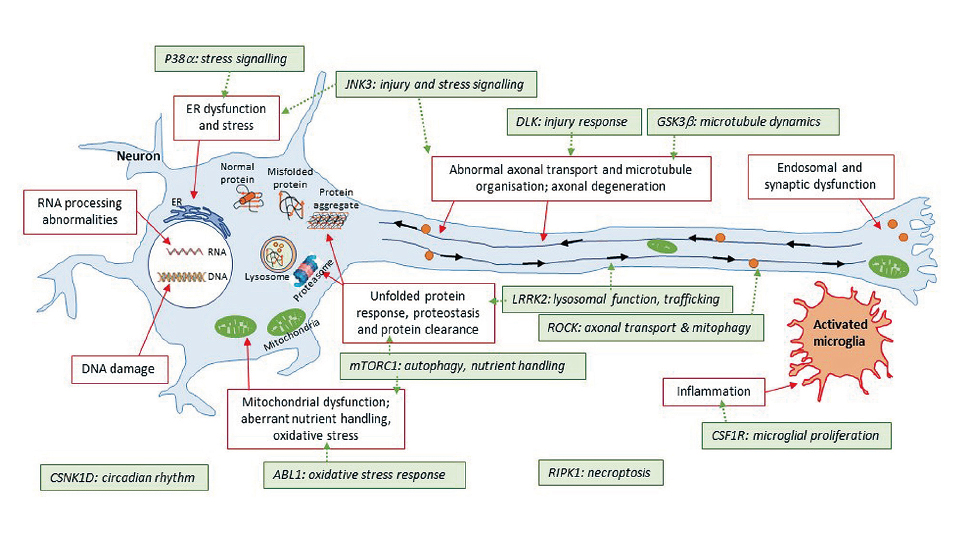
Figure 1. Promising kinase targets and converging pathways in neurodegenerative diseases.
Figure source: https://doi.org/10.3389/fnagi.2020.00242
The Role of Kinases in Neurodegenerative Diseases
Kinases are integral to the regulation of signaling pathways in the brain, and alterations in their function are implicated in neurodegeneration. In neurodegenerative diseases, certain kinases are often overactive or overexpressed in specific brain regions. This dysregulation frequently drives the hyperphosphorylation of proteins that are prone to aggregation, such as tau in AD, α-synuclein in PD, and TDP-43 in ALS 6. Moreover, aberrant kinase signaling can also induce other disease-promoting phenotypes, including neuroinflammation and neuronal death. Conversely, loss-of-function in kinases has emerged as a critical genetic factor and a major contributor to the development of several neurodegenerative diseases.
The two main pathological hallmarks of AD are the extracellular amyloid beta (Aβ) plaques, which result from the accumulation of Aβ, and the intracellular neurofibrillary tangles (NFTs), composed of abnormally phosphorylated tau. Elevated GSK-3β activity is directly linked to tau hyperphosphorylation and increased production, contributing to molecular pathologies, neuronal damage, and cognitive decline in AD 2,3. Fyn also represents a compelling therapeutic target for AD, as it is activated by Aβ through the cellular prion protein (PrPC) and also interacts with tau, uniquely bridging the two key pathologies in AD7.
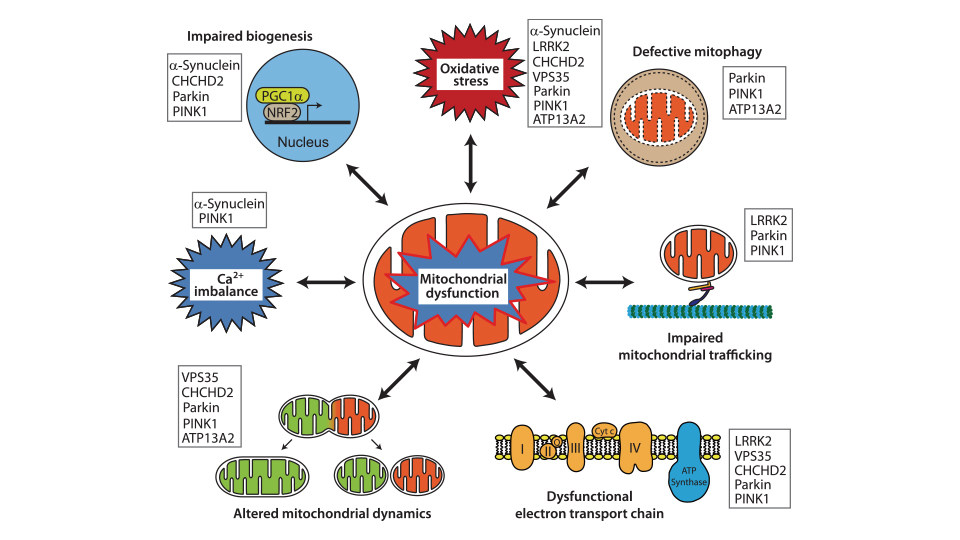
Pathologically, PD is characterized by the progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and the accumulation of intracellular α-synuclein in the form of Lewy-body. Mutations in LRRK2 are the most common genetic cause of familial PD and represent a significant risk factor for sporadic PD 4,8. Mutations in PTEN-induced kinase 1 (PINK1) are also associated with familial PD. Extensive evidence suggests that mitochondrial dysfunction is a central factor in PD pathophysiology (Figure 2). Mutations in autosomal dominant LRRK2 and autosomal recessive PINK1 are directly linked to mitochondrial dysfunction, highlighting their critical roles in the pathogenesis of PD.
Figure 2. Representative pathways of mitochondrial dysfunction implicated in the pathophysiology of PD. The listed proteins contribute pathologically to the different pathways.
Figure source: https://doi.org/10.1007/s11910-018-0829-3
ALS features gradual loss of muscle controls due to degeneration of motor neurons. MAP4K4, a member of the STE20 family, has been identified as a key regulator of motor neuron degeneration in ALS 9,10. Inhibition of MAP4K4 can facilitate neuron survival and prevent neurite degeneration under conditions of exogenous or endogenous stress, suggesting that MAP4K4 is a druggable target for ALS therapeutics.
Development of Kinase Inhibitors for the Treatment of AD, PD, and ALS
In AD: GSK-3β inhibitors have been extensively studied. GSK-3β is involved in tau hyperphosphorylation and Aβ formation, both of which are key pathological features of AD. GSK-3β inhibitors like lithium and other small molecules have shown neuroprotective effects by reducing tau phosphorylation and Aβ levels 3,11. Additionally, c-Jun N-terminal kinase (JNK) inhibitors are being explored for their role in preventing neuronal cell death 11.
In PD: LRRK2 inhibitors are a major focus 8. Mutations in LRRK2 are linked to both familial and sporadic PD, and inhibiting its kinase activity has shown promise in preclinical models 4,8. Several highly selective and potent LRRK2 inhibitors are currently in development, with some advancing to clinical trials. These inhibitors aim to reduce the pathological effects of mutated LRRK2, such as perturbed vesicular trafficking 12.
In ALS: The development of MAP4K inhibitors has gained attention. MAP4K inhibitors, such as Prosetin, have demonstrated neuroprotective effects by protecting motor neurons from endoplasmic reticulum (ER) stress-induced apoptosis 5,9.
Kinase Inhibitors in Preclinical and Clinical trials for Neurodegenerative Diseases
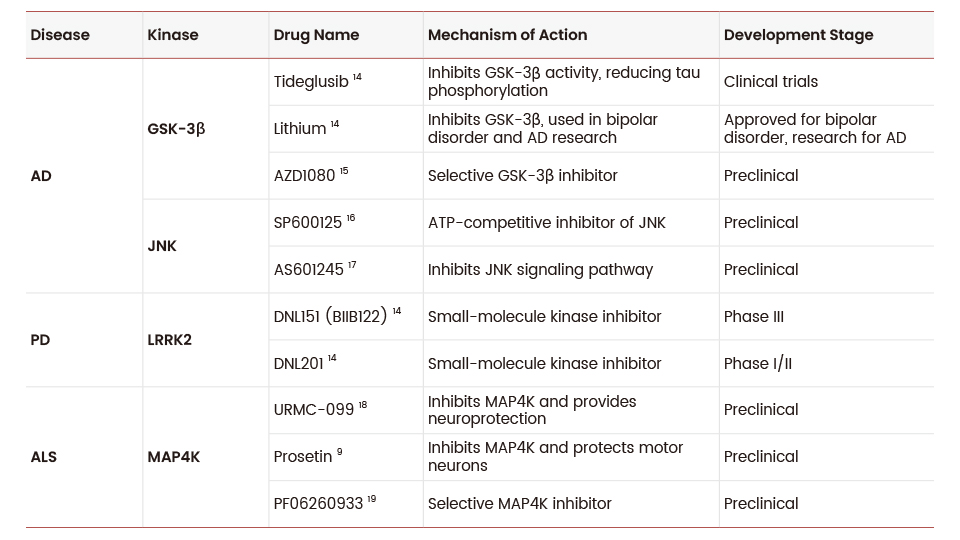
While several kinase inhibitors are in various stages of clinical trials, none have yet received FDA approval specifically for neurodegenerative diseases (Table 1) 13. The development of kinase inhibitors for neurodegenerative diseases has demonstrated promising potential in recent years. These compounds are designed to cross the blood-brain barrier and have shown efficacy in preclinical models, with ongoing efforts to advance them to clinical trials.
Table 1. Kinase inhibitors in preclinical and clinical trials for neurodegenerative diseases
Challenges in Developing Kinase-Targeted Drugs
Developing kinase-targeted drugs for the treatment of neurodegenerative diseases presents several unique challenges. One major issue is achieving specificity. Kinases have highly conserved ATP-binding sites, making it difficult to design inhibitors that selectively target only the desired kinase without affecting others, which can lead to off-target effects and toxicity 20. Additionally, the blood-brain barrier poses a significant obstacle. It restricts the delivery of many potential therapeutic compounds to the brain, necessitating the development of drugs that can effectively cross this barrier1. The complexity of kinase signaling networks in the brain is also a challenge, which means that inhibiting a single kinase may not be sufficient to produce a therapeutic effect. As a result, combination therapies are often required, which can complicate treatment regimens 21. Furthermore, the development of drug resistance is a concern, as cells can adapt to kinase inhibition through various mechanisms, including mutations and activation of alternative pathways 22. These challenges require innovative approaches in drug design, rigorous preclinical testing, and carefully designed clinical trials to develop effective and safe kinase-targeted therapies for neurodegenerative diseases.
Solutions for Kinase Drug Discovery and Neuroscience Research
To support neurodegenerative disease research and kinase inhibitor discovery, SignalChem Biotech (part of Sino Biological) offers comprehensive solutions. SignalChem Biotech is deeply engaged in every crucial aspect of kinase drug discovery by offering the world’s largest portfolio of highly active kinases (700+ wild-type and 400+ mutant kinases), custom enzyme and assay development, and swift compound screening and profiling services. Additionally, SignalChem Biotech has developed an extensive range of therapeutic targets for neurodegenerative diseases, such as the world’s largest recombinant tau protein library, and a plethora of signaling proteins, based on proprietary production and quality control platforms.
Discover our integrated solutions for CNS research and drug discovery.
References:
- Krahn, A. I. et al. Defining the Neural Kinome: Strategies and Opportunities for Small Molecule Drug Discovery to Target Neurodegenerative Diseases. ACS Chemical Neuroscience vol. 11 1871–1886 Preprint at https://doi.org/10.1021/acschemneuro.0c00176 (2020).
- Limantoro, J., de Liyis, B. G. & Sutedja, J. C. Akt signaling pathway: a potential therapy for Alzheimer’s disease through glycogen synthase kinase 3 beta inhibition. Egyptian Journal of Neurology, Psychiatry and Neurosurgery vol. 59 Preprint at https://doi.org/10.1186/s41983-023-00751-2 (2023).
- Llorens-Martín, M., Jurado, J., Hernández, F. & Ávila, J. GSK-3β, a pivotal kinase in Alzheimer disease. Frontiers in Molecular Neuroscience vol. 7 Preprint at https://doi.org/10.3389/fnmol.2014.00046 (2014).
- Taymans, J. M., Baekelandt, V. & Harvey, K. Regulation and targeting of enzymes mediating Parkinson’s disease pathogenesis: Focus on Parkinson’s disease kinases, GTPases, and ATPases. Frontiers in Molecular Neuroscience vol. 7 Preprint at https://doi.org/10.3389/fnmol.2014.00071 (2014).
- Ma, S. & Zhang, C. L. MAP4K inhibition as a potential therapy for amyotrophic lateral sclerosis. Neural Regeneration Research vol. 19 1639–1640 Preprint at https://doi.org/10.4103/1673-5374.389639 (2024).
- Axtman A. D. Characterizing the role of the dark kinome in neurodegenerative disease – A mini review. Biochimica et biophysica acta. General subjects, 1865(12), 130014. https://doi.org/10.1016/j.bbagen.2021.130014 (2021).
- Nygaard, H. B., Van Dyck, C. H. & Strittmatter, S. M. Fyn Kinase Inhibition as a Novel Therapy for Alzheimer’s Disease. http://alzres.com/content/6/1/8.
- Cabezudo, D., Baekelandt, V. & Lobbestael, E. Multiple-Hit Hypothesis in Parkinson’s Disease: LRRK2 and Inflammation. Frontiers in Neuroscience vol. 14 Preprint at https://doi.org/10.3389/fnins.2020.00376 (2020).
- Cobos, S. N. & Torrente, M. P. Epidrugs in Amyotrophic Lateral Sclerosis/Frontotemporal Dementia: Contextualizing a Role for Histone Kinase Inhibition in Neurodegenerative Disease. ACS Pharmacol Transl Sci 5, 134–137 (2022).
- Wu, C., Watts, M. E., & Rubin, L. L. MAP4K4 Activation Mediates Motor Neuron Degeneration in Amyotrophic Lateral Sclerosis. Cell reports, 26(5), 1143–1156.e5. https://doi.org/10.1016/j.celrep.2019.01.019 (2019).
- Zhang, T., Kim, B. M. & Lee, T. H. Death-associated protein kinase 1 as a therapeutic target for Alzheimer’s disease. Translational Neurodegeneration vol. 13 Preprint at https://doi.org/10.1186/s40035-023-00395-5 (2024).
- Kobeissy, F. H. et al. Development of Mutation-Selective LRRK2 Kinase Inhibitors as Precision Medicine for Parkinson’s Disease and Other Diseases for Which Carriers Are at Increased Risk.
- Lui, A. et al. FDA-Approved Kinase Inhibitors in Preclinical and Clinical Trials for Neurological Disorders. Pharmaceuticals vol. 15 Preprint at https://doi.org/10.3390/ph15121546 (2022).
- Home | ClinicalTrials.gov. https://clinicaltrials.gov/.
- Arciniegas Ruiz, S. M. & Eldar-Finkelman, H. Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward. Frontiers in Molecular Neuroscience vol. 14 Preprint at https://doi.org/10.3389/fnmol.2021.792364 (2022).
- Rahman, M., Zhang, Z., Mody, A. A., Su, D. M. & Das, H. K. Intraperitoneal injection of JNK-specific inhibitor SP600125 inhibits the expression of presenilin-1 and Notch signaling in mouse brain without induction of apoptosis. Brain Res 1448, 117–128 (2012).
- Gibbons, G. S., Gould, H., Lee, V. M. Y., Crowe, A. & Brunden, K. R. Identification of small molecules and related targets that modulate tau pathology in a seeded primary neuron model. Journal of Biological Chemistry 299, (2023).
- Bos, P. H. et al. Development of MAP4 Kinase Inhibitors as Motor Neuron-Protecting Agents. Cell Chem Biol 26, 1703-1715.e37 (2019).
- Lasham, D. J. et al. Effects of MAP4K inhibition on neurite outgrowth. Mol Brain 16, (2023).
- Benn, C. L. & Dawson, L. A. Clinically Precedented Protein Kinases: Rationale for Their Use in Neurodegenerative Disease. Frontiers in Aging Neuroscience vol. 12 Preprint at https://doi.org/10.3389/fnagi.2020.00242 (2020).
- Li, S. et al. Signaling pathways in brain tumors and therapeutic interventions. Signal Transduction and Targeted Therapy vol. 8 Preprint at https://doi.org/10.1038/s41392-022-01260-z (2023).
- Grant, S. K. Therapeutic Protein Kinase Inhibitors. Cellular and Molecular Life Sciences vol. 66 1163–1177 Preprint at https://doi.org/10.1007/s00018-008-8539-7 (2009).


 stock.adobe.com/Sadushi
stock.adobe.com/Sadushi