ELEVECTA® Unlocking The Full Potential of Gene Therapy
Gene therapy, considered to be the most efficient and sometimes only treatment option for many severe and life-threatening diseases, is shifting to more common indications with higher numbers of patients including Parkinson's and Alzheimer's disease. This is where the new ELEVECTA® AAV producer cell lines come into play. ELEVECTA® is a revolutionary technology that enables viral vector manufacturing in an industry-standard, scalable suspension format.
CEVEC Pharmaceuticals GmbH, the Germany-based biotech expert in the production of complex biotherapeutics, has recently launched ELEVECTA®, a first-in-class technology featuring stable AAV producer cell lines. The technology addresses one of the biggest hurdles in gene therapy, manufacturing gene therapy vectors with unprecedented robustness, full scalability, and consistent quality.
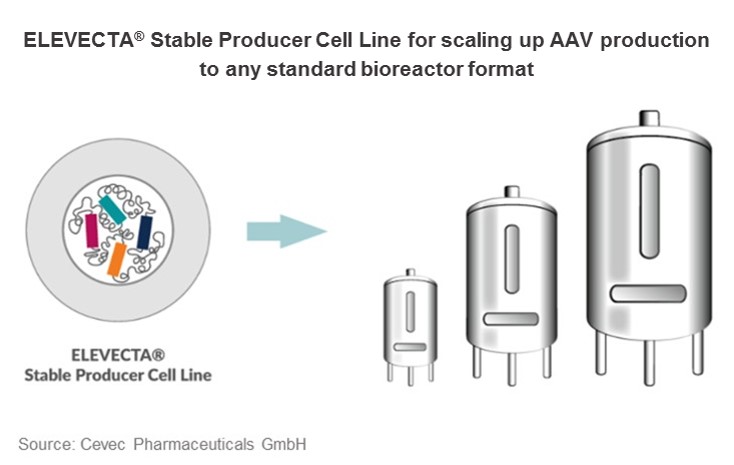
Suspension cell lines for viral vector production at industrial scale
With the newly introduced technology, CEVEC has been successful in generating genuine AAV producer cell lines. ELEVECTA® producer cell lines grow to high densities in serum-free suspension culture. They are easy to handle and adaptable to all current bioreactor formats, enabling industrial scale production of viral vectors comparable to monoclonal antibody production.
No cGMP-grade plasmids required
With the ELEVECTA® platform the industry is moving away from transient transfection towards producer cells with all components required for AAV production integrated into one cell line. No need for expensive cGMP plasmids, special transfection reagents and difficult-to-scale-up transfection protocols. Just one cell line is needed which, after propagation and expansion to the desired cell titers, is switched on to produce and release AAV vectors at high yields into the culture medium.
A modular system to boost standardization
ELEVECTA® is developed as a modular system. AAV vectors can have different surface structures, so called capsids, which in nature allow the virus to target specific cells in a body. For gene therapy applications these capsids are selected or further engineered to improve their tissue-specificity and safety. ELEVECTA® producer cell lines are designed to incorporate any capsid gene of choice, be it natural or engineered. The modularity allows for standardized processes and fast adaption to specific needs.
Unleashing the power of gene therapies
ELEVECTA® sets a new gold standard in vector production for modern AAV-based gene therapies. With its stable producer cells, full scalability, consistent quality and robust processes it provides the efficiency and effectiveness that are needed to pave the way for the success of modern gene therapies in a broad range of indications.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink