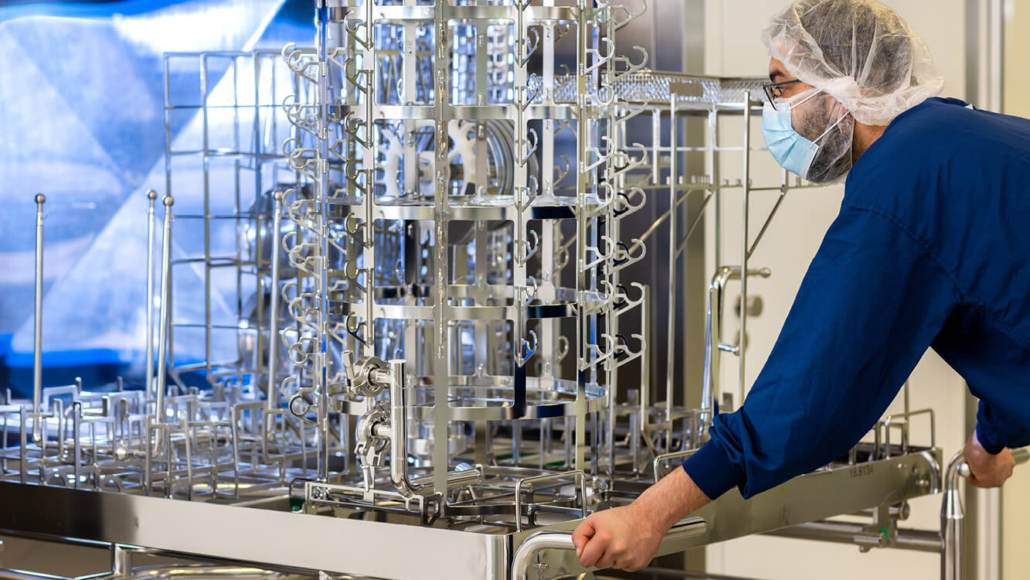
Swiss AB2 Bio inks US$ 686m license option deal with Nippon Shinyaku
Nippon Shinyaku has secured an option to acquire exclusive US rights to commercialize AB2 Bio’s tadekinig alfa to treat primary monogenic IL-18 driven hyperinflammatory syndrome in patients with NLRC4 mutation and XIAP deficiency.
The Swiss EFPL spin-out (2010) AB2 Bio SA got an initial paymant of US$36m including an upfront payment of US$6m upon signature of the licence option agreement with Nippon Shinyaku Co. Ltd. According to the contract, AB2 Bio can gain additional development payments up to US$150m for the Phase III recombinant IL-18 binding protein (tadekinig alfa) that neutralises high blood levels of the proinflammatory cytokine IL-18, which triggers hyperinflammation in patients with NLRC4 mutations and XIAP decifiency. Furthermore, Nippon Shinyaku will pay US$500m in commercial milestones and royalties, if AB2 Bio gets the BLA and marketing authorisation from the US Food and Drug Administration.
If Nippon Shinyaku exercises the option, the company will gain exclusive US commercialisation rights (including Guam, Puerto Rico and U.S. Virgin Islands) to tadekinig alfa as treatment for primary monogenic IL-18 driven hyperinflammatory syndrome in patients with NLRC4 mutation and XIAP deficiency. AB2 Bio retains rights to tadekinig alfa for all other indications globally. Currently, AB2 Bio, runs two Phase II trials in the indications Macrophage Activation Syndrome and adult-onset Still’s disease. Tadekinig alfa has obtained Orphan Drug Designations in both Europe and the US.
Tadekinig alfa, which got a FDA Breakthrough Designation in 2017 in primary hyperinflammatory syndrome, is a novel, recombinant human interleukin-18 binding protein (IL-18 BP) that binds and inhibits IL-18. In healthy people, a large excess of naturally occurring endogenous IL-18 binding protein keeps levels of systemic free IL-18 undetectable. Dysregulation of this balance in autoinflammatory diseases results in high systemic levels of free IL-18 leading to dangerous pathological hyperinflammation. Tadekinig alfa treatment restores the IL-18 BP/IL-18 balance by capturing excess free IL-18, thereby reducing inflammation. This is a novel and promising approach for the treatment of autoimmune diseases and conditions characterised by high systemic IL-18 levels.
Primary monogenic IL-18 driven Hyperinflammatory Syndrome is a potentially life-threatening disease with no approved treatments. If left untreated, the disease may rapidly progress to multiple-organ failure and death. The Phase III program, for which headline result are expected in H1/2025, enrolled patients with verified NLRC4 or XIAP mutations who continued to suffer from severe, life-threatening hyperinflammation despite symptomatic treatment. Mutations in the NLRC4 or XIAP genes are associated with extremely high systemic levels of the pro-inflammatory cytokine IL-18. The excessive release of IL-18 drives the pathology of this clearly defined subgroup of primary hemophagocytic lymphohistiocytosis (HLH). The resulting hyperactivation of immune cells leads to a multiorgan pathology and non-reversible organ damage in fatal cases. This ultra-rare disease occurs most often in infants and young children.
According to a 2020 agreement with AB2 Bio Ltd, Wuxi Biologics is the CDMO set to produce Tadekinig alfa at commercial scale. Though Wuxi Biologics is on the blacklist of the upcoming US BIOSECURE Act, this may have no impact on commercial production of the live-saving drug for children as the BIOSECURE Act only aims to restrict US companies from working with specific Chinese firms, such as WuXi Biologics


 stock.adobe.photo.com/Popelniushka
stock.adobe.photo.com/Popelniushka  Boehringer Ingelheim
Boehringer Ingelheim Adobe stock photos - nadia
Adobe stock photos - nadia