Sphingotec licenses kidney function test
German diagnostics company Sphingotec GmbH has licensed its real-time kidney function test to US diagnostics giant Beckman Coulter allowing it to expand its business to the US as announced earlier this month.
Until now, the Hennigsdorf-based diagnostics company Sphingotec GmbH, which was founded with the proceeds from the sale of B.R.A.H.M.S. to ThermoFisher Scientific, has been under the radar of international diagnostics giants despite great interest from medical opinion leaders. Now, Sphingotec Managing Director Dr. Deborah Bergmann, daughter of company founder and former B.R.A.H.M.S. CSO Dr. Andreas Bergmann, has concluded a licensing deal worth tens of millions of euros allowing the German diagnostics maker make his announcement true to expand into the US market following an €5m Series C financing. This alliance marks the first central laboratory license for a penKid assay and aims to significantly enhance the diagnostic capabilities for acute kidney injury (AKI) globally, by leveraging Beckman Coulter’s global installed base of instruments.
Sphingotec’s CEO Deborah Bergmann, commented, “The development of a penKid-based assay for use on Beckman Coulter’s globally installed immunoassay platforms represents a significant step toward realising our vision of transforming diagnostic innovation into tangible patient benefits. This partnership accelerates our mission to deliver precise, actionable insights to clinicians worldwide, ultimately improving outcomes for patients suffering from acute kidney injury.”
The early detection test based on the real-time kidney function biomarker PenKid will be licensed to diagnostic giant Beckman Coulter Diagnostics Inc, who launched a complete analyser, termed DxI 9000 Access Immunoassay, last year.
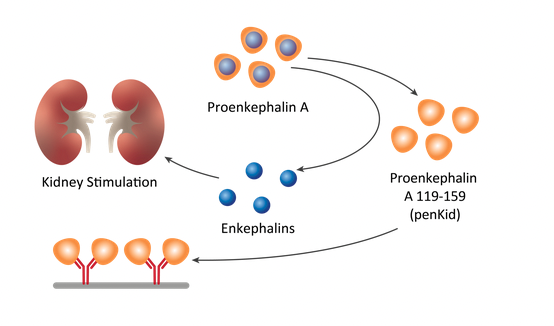
Penkid is a proenkephalin A fragment that is stable in the blood and is a surrogate marker for the peptide hormone enkephalin, which is rapidly degraded and therefore previously unmeasurable, and is released in the event of acute kidney damage. The fact that high penKid levels indicate acute renal dysfunction has been proven by chief intensive care physicians at the University Hospitals of Aachen and Heidelberg, who have been routinely validating the biomarker test since 2020 and 2022 as part of a pilot project. sphingotest® penKid® is a non-automated immunoluminometric assay (ILMA) for the in vitro diagnostic quantitative measurement of Proenkephalin A 119-159 in human EDTA plasma.
Around 20% of patients in intensive care units, an estimated 13 million worldwide, develop acute kidney injury that is often diagnosed too late. The new biomarker enables timely intervention in cases of cardiogenic or septic shock, for example, where kidney failure is a common cause of death.
Under the previous CEO Jörg Menten, the Hennigsdorf-based company had already signed a global license agreement in 2022 and a market launch agreement with South Korean Boditec Med Inc. in August 2023. The Koreans run penKid-Assay on their automated AFIAS platform.
The penKid kidney function test is likely to gain particular market relevance due to the fact that, at almost the same time, scientists reported on a new treatment option for acute kidney failure in the New England Journal of Medicine. They were able to reduce the extent of kidney damage by administering amino acids intravenously.


 gov.uk
gov.uk Gilead Science
Gilead Science stock.adobe.photo.com/Popelniushka
stock.adobe.photo.com/Popelniushka