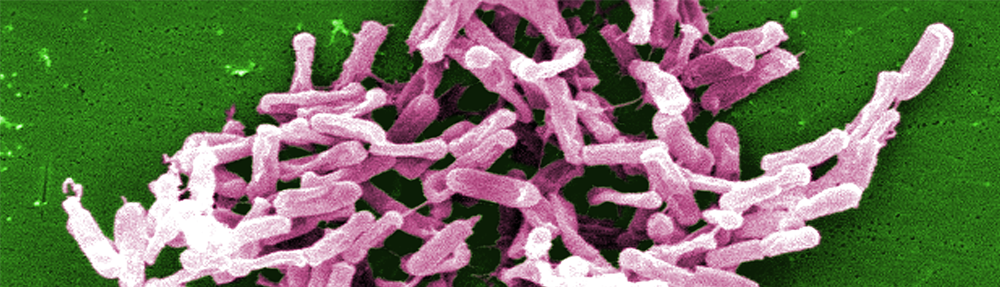
Sanofi Pasteur ends Clostridium difficile vaccine programme
The decision of the vaccine arm of French drug giant Sanofi was based on an independent interim analysis of a pivotal Phase III study enroling up to 16,500 people at risk of Clostridium difficile infection (CDI) for vaccination with the jab that has FDA Fast Track designation.
The probability that the study will meet its primary objective is low, said Sanofi Pasteur in a press release. With the Cdiffense Phase III trial, Sanofi Pasteur tried to proove that its inactivated toxoid vaccine induces production of antibodies directed against C.diff toxins A and B and that this is linked to prevention of of primary symptomatic CDI. Primary symptomatic CDI is defined as a change in bowel habits with passage of three or more loose stools per day for one or more days and either a positive C. diff stool toxin test (A, B or both) or a positive stool cytotoxicity assay and absence of another identified cause for diarrhea. The study’s primary endpoint was the number of symptomatic PCR-confirmed primary C. difficile infection (CDI) cases after at least one injection of the jab.
According to estimates, C. difficile is emerging as a leading microbial cause of healthcare-associated infections found in US hospitals driving up costs to $4.8bn each year in excess health care costs in acute care facilities alone. The study, started in August 2013 and designed to run until October 2019, was in advanced stage. Sanofi Pasteur said data from all vaccinated volunteers will continue to be analysed for more information and shared with the scientific community but did not specifiy a publication date.
The single-blind, placebo-controlled, international trial evaluated single injections given on days 0, 7 and 30 in about 16,500 adult volunteers at least 50 years old who are planning an upcoming hospitalization or have had at least two hospital stays and have received systemic antibiotics in the past year. Secondary endpoints include the number of PCR-confirmed primary CDI cases after two and three injections, severe PCR-confirmed primary CDI cases, maximum number of loose stools per day, CDI episode/illness duration, immunogenicity and injection site and systemic reactions.
As an alternative treatment, stool transplants have proved to cure 91% of CDI cases in a high number of patients and are currently also be tested in Phase III studies.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink