Sanofi gets the EU’s ok for caplacizumab
The EU approved Sanofi's Cablivi (caplacizumab) as the first antibody therapeutic to treat acquired thrombotic thrombocytopenic purpura (aTTP) on Friday.
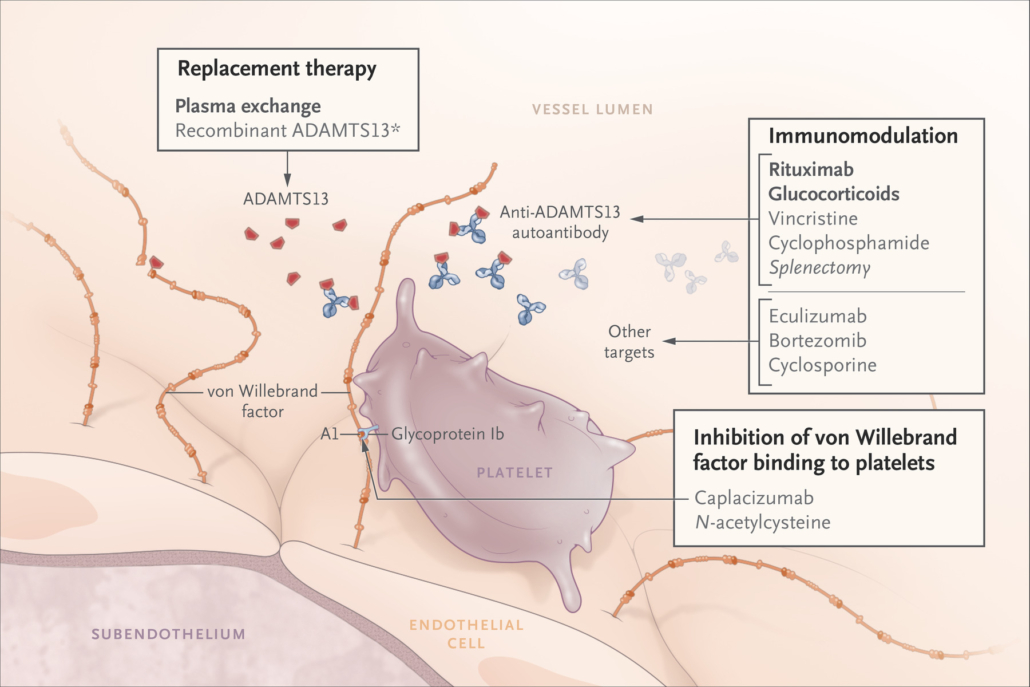
The life-threatening, autoimmune-based blood clotting disorder leads to severe thrombocytopenia, microangiopathic hemolytic anemia, ischemia and organ damage, particularly of the brain and heart. The European Commission limited the scope of application to adults experiencing an aTPP episode. About 20% of patients receiving daily plasma exchange plus immunosuppressors as standard treatment do not survive an episode of aTTP, most of deaths occurring within 30 days of diagnosis.
"The approval of Cablivi provides an important addition to the standard-of-care treatment for patients with aTTP in Europe because it can significantly reduce time to platelet count normalisation and induce a clinically meaningful reduction in recurrences." commented Marie Scully, M.D, professor of hematology at University College London Hospitals
Caplacizumab, is a a humanised bivalent nanobody that prevents von Willebrand factor-mediated platelet adhesion and concomitant reduction of blood clotting factor VIII. The nanobody was developed by Belgian Ablynx NV, which Sanofi acquired early this year. Sanofi Genzyme, the orphan drug arm of Sanofi, will market the drug. A BLA submitted to the US FDA is under priority review. The target action date for the FDA decision is February 6, 2019. Sanofi said, the approval of caplacizumab was a first step in the company’s plan to become a leader in the treatment of rare blood disorders. Earlier this year, Sanofi acquired Bioverativ for €11.8bn, which has treatments for hemophilia A and B.
The approval of caplacizumab in the EU is based on the Phase II TITAN and Phase III HERCULES studies in 220 adult patients with aTTP. The efficacy and safety of caplacizumab in addition to standard-of-care treatment, daily PEX and immunosuppression, were demonstrated in these studies. In the HERCULES study, treatment with caplacizumab in addition to standard-of-care resulted in a significantly shorter time to platelet count response (p<0.01), the study’s primary endpoint; a significant reduction in aTTP-related death, recurrence of aTTP, or at least one major thromboembolic event during study drug treatment (p<0.0001); and a significantly lower number of aTTP recurrences in the overall study period (p<0.001). Importantly, treatment with caplacizumab resulted in a clinically meaningful reduction in the use of PEX and length of stay in the intensive care unit (ICU) and the hospital, compared to the placebo group.
In clinical trials, the most frequently reported adverse reactions were epistaxis, headache and gingival bleeding. No deaths were reported during study drug treatment in the caplacizumab group in the TITAN and HERCULES studies, while for the placebo group, two deaths were reported in the TITAN study and three deaths in the HERCULES study.


 Sitryx Therapeutics
Sitryx Therapeutics
