Roche acquires Telavant Holdings to win TLA1 race
Analysts describe as sporting the €7.1bn for Roche AG's acquisition of Roivant subsidiary Telavant Holdings.
The takeover of Telavant, a 75:25 jont-venture of Roivant Sciences and the US pharmaceutical group Pfizer, however, may cost Roche up to US-$7.25bn. The Basel-based pharma giant cites the excellent Phase IIb data of the anti-TLA1 antibody RVT-3101 in 245 patients with ulcerative colitis, 36% of whom showed clinical remission, as the reason for the investment. A Phase II study in Crohn’s disease is ongoin. Roche sees expansion opportunities in other autoimmune indications in addition to the billion-dollar potential in Crohn’s disease and ulcerative colitis.
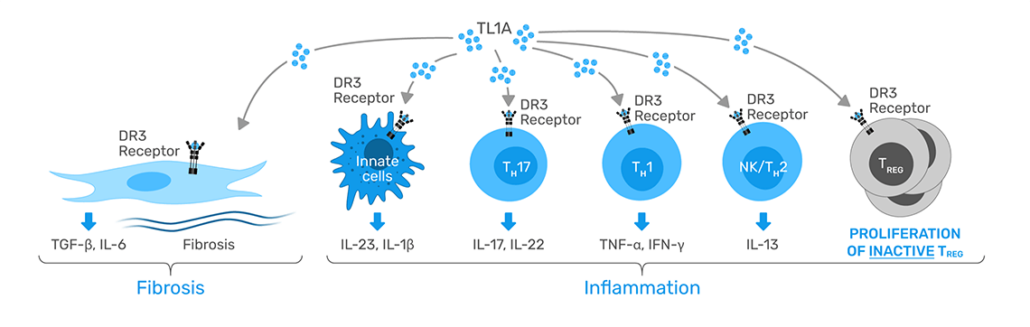
RVT-3101 is a potential first-in-class agent that targets both inflammatory and fibrotic pathways by inhibiting tumour necrosis factor-like cytokine 1A (TL1A). It has been shown to modulate the severity of inflammation and fibrosis by stimulating the TH1 and TH17 pathways, in addition to activating fibroblasts. As such, RVT-3101 has the potential to provide greater efficacy by hitting multiple inflammatory and fibrotic pathways. Roche will also obtain an option to enter into a global collaboration with Pfizer on a next-generation p40/TL1A directed bispecific antibody, currently in Phase 1.
Under the agreement, Roche is paying US-$7.1bn upfront and a short-term milestone payment of US-$150m for the rights to develop, manufacture and commercialise RVT-3101 in the US and Japan. Roche is committed to starting a global Phase III trial for RVT-3101 as soon as possible to bring this promising therapy to the patients suffering from inflammatory bowel disease. Outside of the US and Japan, Pfizer holds commercialisation rights.
Merck Sharp & Dome in June acquired Prometheus Biosciences for US-$10.8bn, and announced to kick-off a Phase III trial of ts anti-TLA1 antibody PRA-023 (now MK-7240) by end of October in 1,020 patients with ulcerative colitis. At the beginning of October, French Sanofi SA entered an US-$1.5bn partnership – including an upfront paymeTeva’s iPhase IIb-Anti-TLA1 programme TEV’574 to Phase III testing and market approval. A hot race has begun and Roche, which wants to start a Phase III trial as soon as possible, is at least catching up with its competitor Merck who wants to close its Phase III study by December 2026.


 Unsplash+
Unsplash+