Pilatus Biosciences Enters Clinical Collaboration with Roche
Pilatus Biosciences AG, a Swiss biotechnology company headquartered in Epalinges with operations in the United States and South Korea, has entered into a clinical trial collaboration with Roche AG. As part of the agreement, Roche will provide its PD-L1 immune checkpoint inhibitor atezolizumab for use in a Phase I study evaluating the combination of atezolizumab with Pilatus’s investigational CD36 inhibitor, PLT012, in patients with hepatocellular carcinoma (HCC).
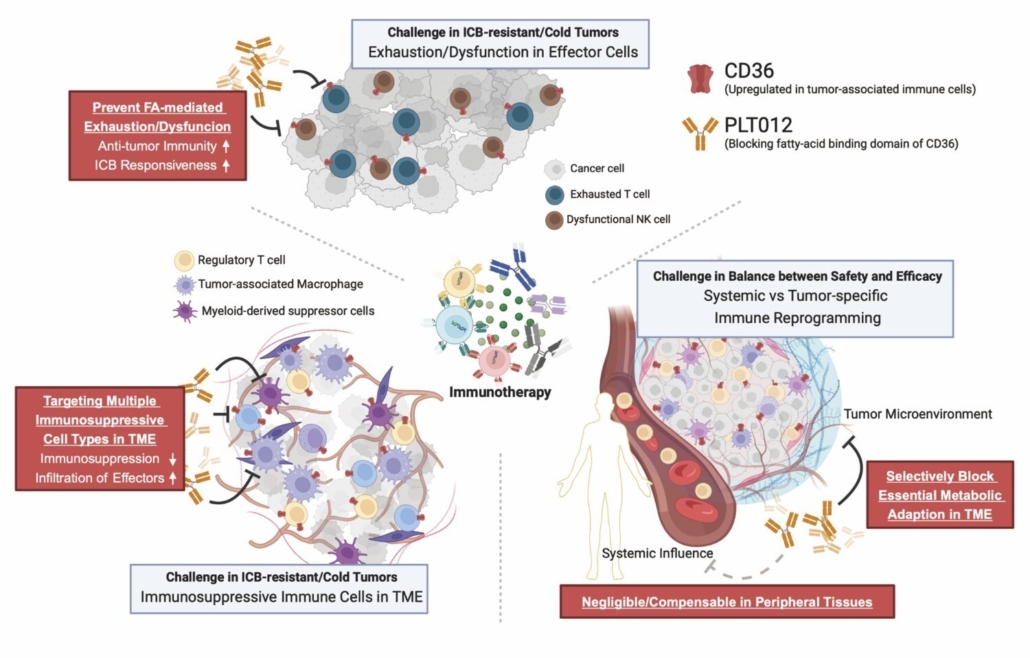
PLT012 is Pilatus’s lead immunomodulatory candidate. It is designed to reprogramme the fibrotic and immunosuppressive tumour microenvironment (TME) characteristic of HCC. CD36, a lipid scavenger receptor, is upregulated in immune cells exposed to lipid-rich, inflammatory environments and is considered a key driver of immune suppression in solid tumours. By blocking CD36-mediated lipid uptake, PLT012 reduces the number of intratumoural regulatory T cells (Tregs) and pro-tumour macrophages, while enhancing infiltration, persistence, and cytotoxic function of CD8-positive T cells.
“Current treatments, including checkpoint inhibitors as monotherapy, often fail to produce durable responses in liver cancer,” said Dr Raven Lin, Chief Executive Officer of Pilatus Biosciences. “The ability of PLT012 to remodel the TME may enhance immune activation and improve therapeutic outcomes.”
In preclinical studies, PLT012 has demonstrated strong monotherapy efficacy in models of liver malignancies, along with a favourable safety profile across species. Its novel mechanism of action also positions it as a potent sensitiser in combination with anti–PD-L1 therapies, potentially overcoming resistance in immunologically ‘cold’ tumours and liver metastases.
Pilatus, which was founded in 2022 as a spin-out from the Ludwig Institute for Cancer Research, operates in the emerging field of immunometabolic oncology. It faces competition from companies targeting the same molecular pathway, notably Spain’s Ona Therapeutics and Distillery Therapeutics. Ona has advanced humanised anti-CD36 antibodies through extensive preclinical evaluation for cancer therapy. Distillery Therapeutics has reported inhibition of tumour growth in melanoma and colorectal cancer models using anti-CD36 monoclonal antibodies, with enhanced anti-tumour responses observed when combined with anti–PD-1 therapy.


 BioDlink
BioDlink Araris Biotech AG
Araris Biotech AG Roche
Roche