Ortho markets predictive heart failure re-hospitalisation test
In vitro diagnostics leader Ortho Clinical Diagnostics (Raritan, US) expands his assay portfolio with Sphingotec's first-in-class biomarker test that predicts residual edema and re-hospitalization in patients with congestive heart failure.
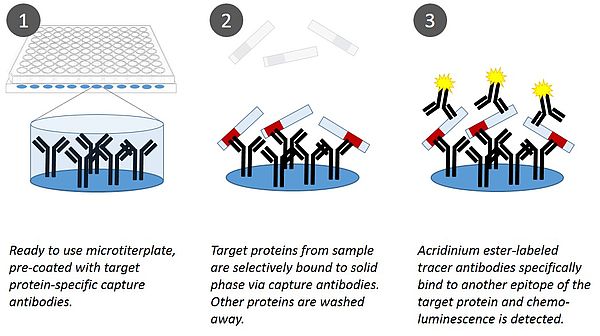
Ortho and sphingotec GmbH today announced a global strategic agreement under which the companies will offer automated testing of adrenomedullin, a first-in-class endothelial function marker predicting re-hospitalisation of heart failure patients suffering from residual edema. Until today, physicians were not able to predict what heart failure patients are therapy-resistant to loop diuretics and thus relapse because of residual vascular leakage/edema. Sphingotec’s sphingotest® bio-ADM® is the very first in vitro diagnostic test that measures blood levels of the endothelial function marker adrenomedullin that prevents vascular leakage and maintains endothelial integrity. Sphingotest® bio-ADM thus allows stratification of patients with acute heart failure who require further diuretics therapy in order to prevent relapse from those who fully recovered under therapy. Edema prediction in heart failure patients is expected to save health systems billions of US-dollars by preventing complications arising from therapy resistance to diuretics.
Under the global agreement, Ortho will use the sphingotest® bio-ADM® on its automated VITROS® platforms and integrated systems for small, medium and high sample throughput clinical laboratories. Product availability is subject to local regulatory requirements.
"Ortho is committed to continually expanding our test menus through internal research and development programmes as well as strategic collaborations," explained Robert Yates, Chief Operating Officer at Ortho. "Our strategic agreement on the sphingotest® bio-ADM® assay enables us to bring this novel cardiac biomarker to a broad range of clinical laboratories around the world."
Edema in acute heart failure is a serious medical problem and a major factor in re-hospitalization in patients with decompensated congestive heart failure. In addition to the widespread use of natriuretic peptides, primarily for the diagnosis of heart failure, the precise determination of residual edema during and after treatment is of great importance in assessing the success of acute heart failure therapy.
bio-ADM® has been studied in numerous clinical trials and has demonstrated its ability to accurately identify residual edema, verify the efficacy of the treatment, and secure the discharge decision.
"Working with Ortho Clinical Diagnostics will help us deliver our standardized sphingotest® bio-ADM® assay to laboratories around the world to identify patients with heart failure and residual edema in a timely manner. bio-ADM® can thus support decisions for meaningful intervention, including the possible adaptation of the medication", said Andreas Bergmann, founder and CEO of sphingotec GmbH.



 SANOFI
SANOFI